Tractional retinal detachment (TRD) is a vision-threatening complication of proliferative diabetic retinopathy. Developing the skills and experience to effectively navigate through these tough cases while avoiding complications is time-consuming. We present the surgical repair of an advanced complex diabetic TRD case with severe fibrovascular proliferation.
Case Presentation
A 55-year-old male with diabetes experienced chronic vision loss in the left eye. His VA was light perception. Clinical examination revealed active proliferative diabetic retinopathy with macula-involving TRD with severe fibrovascular plaque covering the entire posterior pole (Figure 1).
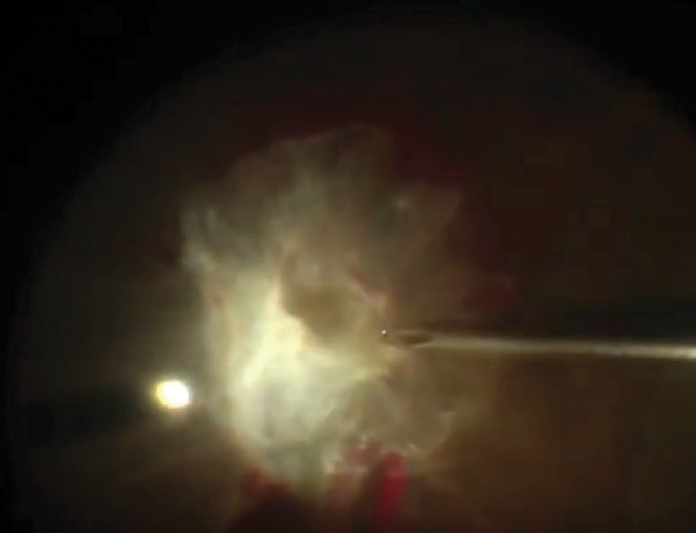
Figure 1. Intraoperative image of the initial presentation of the left eye demonstrating a large central fibrovascular plaque covering the entire posterior pole.
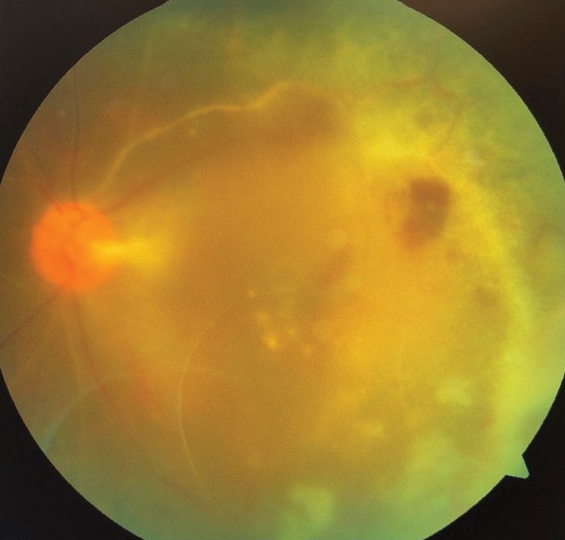
Figure 2. Postoperative fundus photograph showing attached retina.
We performed vitrectomy using the CONSTELLATION Vision System (Alcon) with 23-gauge valved cannulas. Intravitreal bevacizumab (Avastin, Genentech) was injected 3 days prior to surgery. The essential first step was to release anterior-posterior traction of the hyaloid. Using ILM forceps, we searched for the surgical plane and the edges of the membrane. The plaque was fused at the nerve and the macula, so we started dissection at the superior edge where a plane could be initiated.
To start and extend the initial plane toward the center of the plaque, we used 23-gauge vertical membrane peeler-cutter scissors. To segment, delaminate, and remove the membranes, we used the vitrector, along with a blunt delaminating spatula, and vertical scissors to assist with correct plane identification and safe delamination. We left residual pegs in areas of strong focal adhesion where traction was released.
Triamcinolone was injected after membrane peeling, and residual hyaloid was removed using the vitrector and a 23-gauge flex loop membrane scraper (Finesse, Alcon). In locations where iatrogenic breaks were created temporally, we performed thorough peeling of the membranes around the breaks. In the superotemporal area where the membrane adjacent to the breaks was too adherent to peel, we performed small focal retinectomy and carefully marked the breaks with diathermy.
Fluid-air exchange was performed, and laser was applied around the breaks and in panretinal photocoagulation fashion all the way to the ora, using scleral depression. Subsequently, 16% C3F8 gas was used as tamponade.
The retina has remained attached for more than 2 years with this single surgery. The patient’s IOP is normal, and his vision improved and has stabilized to counting fingers at 5 feet (Video).
Dimitra Skondra, MD, PhD, performs a surgical repair of a tractional retinal detachment (TRD) in a diabetic patient who received a bevacizumab (Avastin, Genentech) injection 3 days prior.
Discussion
A complex TRD repair is a true test of fortitude for any vitreoretinal surgeon, as it is one of the most technically challenging scenarios we face. Factors involved in this complexity include friable ischemic retina, fibrovascular membranes adherent to retinal vessels and tissue, adherent hyaloid, intraoperative bleeding, and compromised surgical view because of poor dilation, corneal edema, and cataract.
In our practice, we routinely use intravitreal bevacizumab 2 to 4 days prior to vitrectomy to minimize intraoperative bleeding. In a meta-analysis, Zhao and colleagues found that anti-VEGF pretreatment was associated with decreased intraoperative bleeding duration, iatrogenic retinal breaks, silicone oil use, and relaxing retinotomy.1
Good surgical view is crucial for these challenging cases, given that peeling close to retinal vessels and macula is necessary. We have a low threshold for cataract surgery prior to vitrectomy, and we avoid combined phacoemulsification/vitrectomy for complex TRD cases. Intraoperatively, we apply 50% dextrose on the cornea, and we use viscoelastic instead of hydroxypropyl methylcellulose (Goniosol, Novartis) to maintain corneal clarity.
Using the right tools to remove tractional forces is key. We use the vitrector handpiece (23-gauge or, more recently, 27-gauge handpiece in hybrid 23/27-gauge technique) along with delaminating blunt spatula, vertical membrane peeler-cutter scissors, and ILM forceps for thorough segmentation, delamination, and peeling. A high-magnification macular lens can be useful for better visualization of the surgical plane during peeling.
Patience and persistence are required for meticulous removal of fibrovascular membranes to relieve tractional forces. According to Khan and colleagues,2 27-gauge pars plana vitrectomy is shown to have low rates of postoperative complications in primary and complex retinal detachments; however, Storey and colleagues3 found that instrument gauge had no impact on anatomical or visual outcomes in diabetic TRD repair.
Peeling every membrane completely is not necessary; residual pegs can be left, as long as traction is released. If breaks are identified, adjacent membranes at the edge of a break should be removed. If they are too adherent and traction is still present, focal retinectomies can be considered. It is important to remove as much of the residual hyaloid to eliminate the scaffold for reproliferation postoperatively. There is usually more hyaloid than it appears, so staining with triamcinolone after membrane peeling is helpful.
We use long-acting 14% or 16% C3F8 gas tamponade with prolonged face-down positioning in more than 95% of TRD cases without breaks or even in the presence of multiple breaks. We rarely use silicone oil, using it only if an extensive 180º inferior retinectomy is needed (about 5% of cases). This gas tamponade/face-down positioning approach has provided excellent results: an approximately 98% primary reattachment rate with a single surgery and less than 2% secondary retinal detachments in complex cases.4 The tamponade agent has been found to be associated with anatomical success as well.3,5,6
Conclusion
TRD repair is among the most complex intraocular surgeries. The evolution of small-gauge vitreoretinal instrumentation along with our surgical skills and thoughtful preoperative, intraoperative, and postoperative measures combine to significantly improve surgical outcomes.
1. Zhao XY, Xia S, Chen YX. Anti-vascular endothelial growth factor agents pretreatment before vitrectomy for complicated proliferative diabetic retinopathy: a meta-analysis of randomised controlled trials. Br J Ophthalmol. 2017. pii: bjophthalmol-2017-311344. [Epub ahead of print]
2. Khan MA, Kuley A, Riemann CD, et al. Long-term visual outcomes and safety profile of 27-gauge pars plana vitrectomy for posterior segment disease. Ophthalmology. 2018;125:423-431.
3. Storey PP, Ter-Zakarian A, Philander SA, et al. Visual and anatomical outcomes after diabetic traction and traction-rhegmatogenous retinal detachment repair. Retina. 2017.
4. Dawood S, Georgiou M, Patel K, Skondra D. Outcomes of diabetic tractional retinal detachment repair In Chicago Cook County Hospital Health System: initial experience of a new service. Poster presented at: ARVO; May 2016, Seattle, WA.
5. Mikhail M, Ali-Ridha A, Chorfi S, Kapusta MA. Long-term outcomes of sutureless 25-G+ pars-plana vitrectomy for the management of diabetic tractional retinal detachment. Graefes Arch Clin Exp Ophthalmol. 2017;255:255-261.
6. Shen YD, Yang CM. Extended silicone oil tamponade in primary vitrectomy for complex retinal detachment in proliferative diabetic retinopathy: a long-term follow-up study. Eur J Ophthalmol. 2007;17:954-960.



