For the vast majority of my patients across a number of disease states, I order OCT angiography (OCTA) on the ZEISS PLEX Elite. There are a few exceptions: I reserve fundus photography for cases that require multimodal imaging and usually order fluorescein angiography (FA) with indocyanine green for uveitis patients. Still, OCTA has become one of my primary tools, and I have found that by learning to read flow void patterns near choroidal neovascular membranes and by understanding the differences between inflammatory lesions and choroidal neovascular membranes on imaging, I have been able to expand my knowledge of my patients’ diseases and tailor my treatments to their specific needs.
In this article, I share images that illustrate how the PLEX Elite has informed my decision-making.
DIABETIC EYE DISEASE
A 35-year-old pregnant woman with type 1 diabetes mellitus presented to my clinic with floaters and for evaluation of diabetic retinopathy (DR). OCTA montage imaging on the PLEX Elite demonstrated extensive proliferative DR (Figure 1). Based on this test, I assessed the patient’s degree of neovascularization and proliferation and observed extensive ischemia in the periphery.
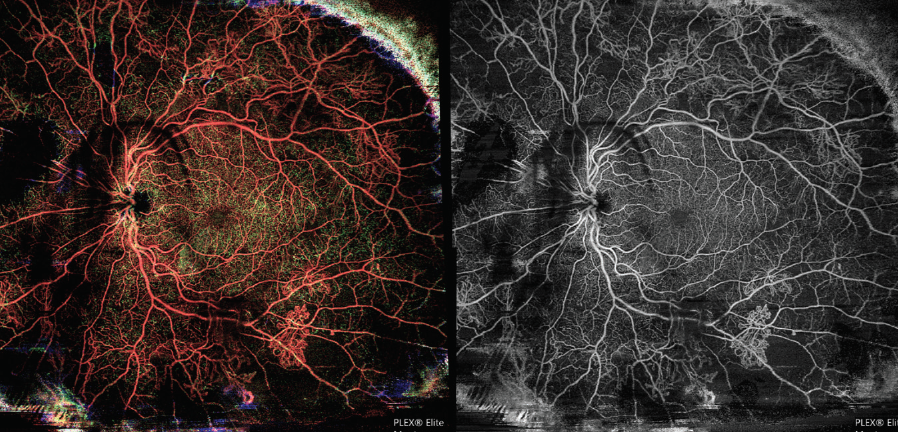
Figure 1. OCTA montage shows peripheral ischemia and extensive proliferative DR in this 35-year-old pregnant patient.
I find that in patients with diabetic eye disease who struggle with metabolic control and in pregnant patients for whom FA is a relative contraindication, OCTA readouts such as this one are useful teaching tools. Some patients find an easy-to-understand imaging report instructive. In those patients, I can hammer home a message about the relationship between their diabetes and their visual outcome.
SICKLE CELL RETINOPATHY
My research group at Columbia University is studying the effect of bone marrow transplantation and sickle cell retinopathy in the pediatric and adolescent population. In our first publication, we concluded that OCTA is an effective tool for detecting sickle cell retinopathy in adolescent populations, describing the platform as allowing “more sensitive visualization of retinal thickness and blood flow through the deep and superficial plexuses in the macular region compared with FA.”1 Further, we also found that, “in contrast to ultrawide-field FA, which requires dye injection and produces a 2D image, OCTA is noninvasive and allows visualization of all three major capillary networks (superficial retinal, deep retinal, and choriocapillaris).”1
Importantly, this study found that OCTA detected flow voids in the superficial and deep retinal capillary plexuses in the macula of children.1 The study showed that sickle retinopathy creates macular ischemia earlier than we previously thought. Given the noninvasive nature of OCTA and the evidence that it can be used to identify disease activity, this platform is particularly well suited for evaluating anatomy in patients with sickle cell retinopathy.
Figure 2 shows B-scans (A) and OCTA of the superficial (B) and deep (C) plexuses of a 12-year-old boy with sickle cell disease Hb SS. Flow voids are observed on the OCTA reports. Interpretation of B-scan imaging is informed by OCTA imaging. When I observe certain patterns on a B-scan (ie, temporal thinning), I know that these areas correspond to flow voids on OCTA. I have a more nuanced appreciation for ischemic disease when reports from these two modalities are viewed side-by-side.
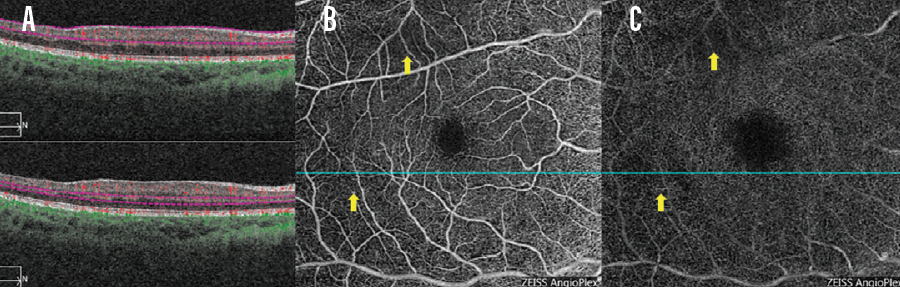
Figure 2. A 12-year-old boy presented with sickle cell disease Hb SS. OCT B-scans (A) and OCTA of the superficial (B) and deep (C) plexuses are seen here. Flow voids are observed on the OCTA reports (B, C, yellow arrows), which in turn inform interpretation of the B-scan.
AGE-RELATED MACULAR DEGENERATION
OCTA has demonstrated utility for managing cases of neovascularization and both wet and dry age-related macular degeneration (AMD). B-scans offer guidance regarding treatment decisions, and OCTA helps me distinguish lesion type and size.
A recent case from my clinic illustrates this well. An 81-year-old woman with a history of monthly intravitreal injections of aflibercept (Eylea, Regeneron) presented to the clinic. Examination of her right eye (OD) and left eye (OS) revealed VA of 20/30 and 20/80, respectively.
OCTA helped me identify the area of neovascularization OD, as well as the presence of flow voids around the neovascular area (Figure 3A). The OCTA report OS may lead the untrained observer to conclude that a neovascular membrane is present OS (Figure 3B). However, the corresponding OCT scan demonstrates that this is a geographic atrophy lesion. The OCTA image also lacks the characteristic flow void typically found around choroidal neovascular lesions.
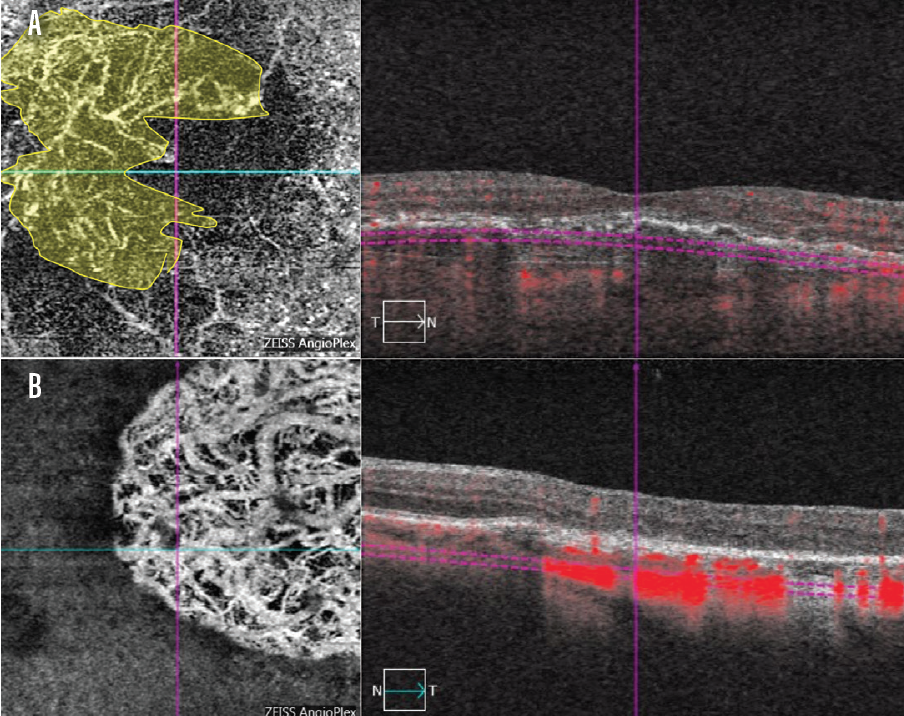
Figure 3. On OCTA, the area of neovascularization is nicely delineated OD in a patient with neovascular AMD (A, area in yellow). OCTA helped determine that a geographic atrophy lesion, rather than neovascularization, was present OS (B).
Comparing the OCTA image of a patient without dry AMD (Figure 4A) to images from dry AMD patients (Figure 4B, C, D) demonstrates how OCTA detects areas of disease. Often, these lesions are found in the choriocapillaris and are not detected in the deeper layers of the choroid. In patients with evidence of dry AMD in the choriocapillaris, it is unclear if flow voids arise due to the presence of drusen or if drusen are a risk factor for flow void genesis.
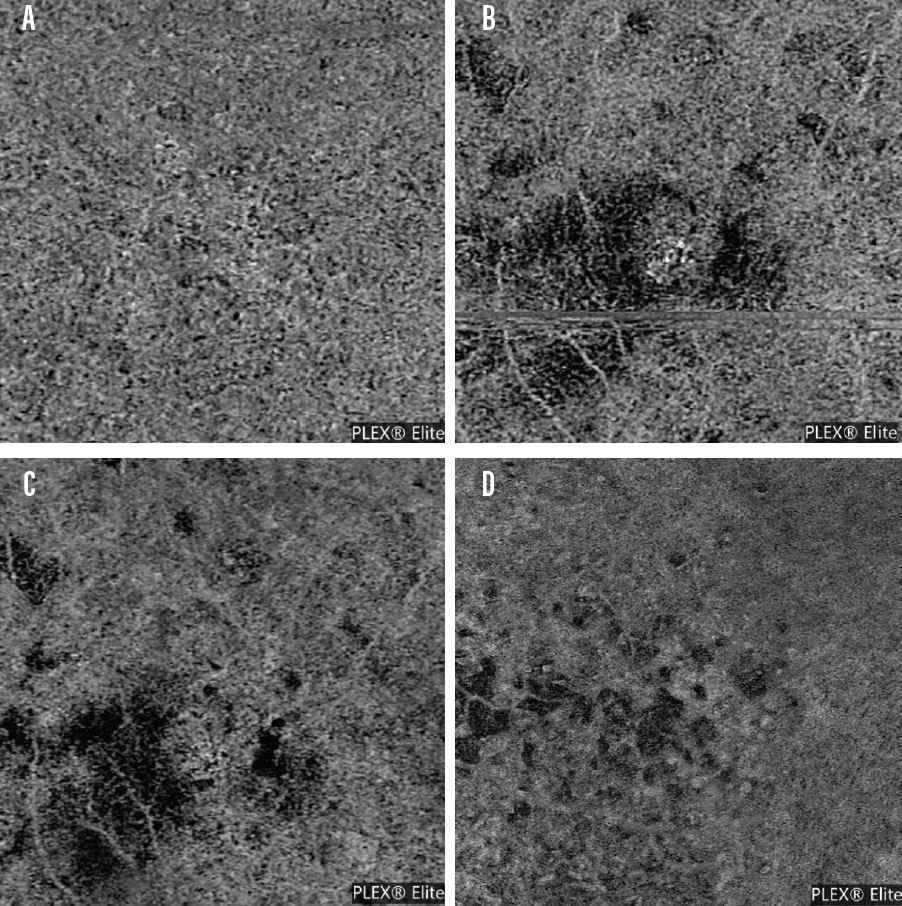
Figure 4. An OCTA scan from the PLEX Elite of a patient without evidence of dry AMD (A) and OCTA scans of patients with dry AMD as detected on imaging (B-D). Patients with dry AMD often present with various flow void patterns.
POLYPOIDAL CHOROIDAL VASCULOPATHY
Detection of polypoidal choroidal vasculopathy (PCV) is enhanced on the PLEX Elite. In the case of a 43-year-old woman with PCV, I noted subretinal fluid and a choroidal neovascular membrane beneath the retinal pigment epithelium on B-scan (Figure 5A). A large branching vascular network was imaged on OCTA (Figure 5B). Because of the clarity with which I could follow the neovascular membrane, I determined that FA imaging was unnecessary. Some other cuts on the OCTA also demonstrated the typical terminal polypoidal changes, and I therefore spared this patient invasive contrast-based imaging.
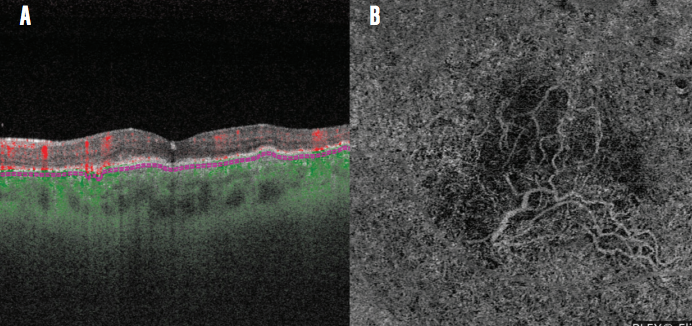
Figure 5. Neovascular membrane and subretinal fluid are imaged on OCT B-scan (A) and a branching network is observed on OCTA (B) in this patient with PCV.
CONCLUSION
When considering the range of tests we can order for patients, we must prioritize invasiveness, speed, and accuracy. Imaging with the PLEX Elite keeps my patients comfortable; allows me to understand nuances about their particular disease, which in turn allows patient-specific care; and provides imaging results that my patients and I can easily interpret.
1. Pahl DA, Green NS, Bhatia M, et al. Optical coherence tomography angiography and ultra-widefield fluorescein angiography for early detection of adolescent sickle retinopathy. Am J Ophthalmol. 2017;183:91-98.

