In 2011, BEAT-ROP became the first randomized, multicenter trial of anti-VEGF therapy in ROP, reporting significant treatment effects for bevacizumab over laser therapy for Zone 1 (but not Zone 2) disease in infants with Stage 3+ ROP.28 However, BEAT-ROP left some unresolved questions regarding the appropriate dose for anti-VEGF therapy in infants with ROP.31 BEAT-ROP used half the dose of bevacizumab usually used (off-label) in adults with retinal disorders, but without conducting dose-finding studies. The majority of infants with ROP require bilateral treatment for ROP, so according to the BEAT-ROP protocol, they ultimately received a total dose of bevacizumab therapy equivalent to that which an adult receives (off-label) for unilateral treatment.31 “There’s certainly room for discussion here as to whether this is the right dose,” said Prof. Andreas Stahl. “Another open question is that of systemic safety. We know from studies in infants with ROP that an ocular injection of bevacizumab reaches the circulation and suppresses VEGF, not only in the eye, but also systemically.32”
In order to collect further evidence on the use of anti-VEGF therapy in infants with ROP, the German Retina.net ROP registry was set up in 2012 with the aim of capturing treatment patterns and outcomes.33,34 It now includes data from over 330 treated patients, which corresponds to around 10 to 15% of all treated ROP infants in Germany.34 Analysis of the registry data reveals that treatment patterns have changed over time in Germany, with anti-VEGF treatment given to 10% of treated eyes in 2011 but to 56% and 30% in 2014 and 2015, respectively (Figure 7).34 Almost all eyes with AP-ROP or Zone 1 disease received anti-VEGF treatment, while Zone 2 disease was predominantly treated with laser.34 “We’re currently looking at the data from 2016 and onwards to see how these trends developed further in the following years,” said Prof. Stahl.

Figure 7. Treatment patterns in the Retina.net ROP registry.
More recently, the CARE-ROP study was an interventional investigator-initiated study performed to investigate the use of ranibizumab in 19 infants with ROP, given at two different doses: 0.12 mg and 0.20 mg, corresponding to 24% and 40% of the adult dose.35 CARE-ROP was a blinded, randomized, multicenter study performed in Germany, with the primary endpoint (the proportion of infants who did not require rescue therapy) at 24 weeks and follow-up ongoing to 5 years.35 In the group who completed the study, 88.9% of infants (94.4% of eyes) in the 0.12-mg group and 85.7% of infants (92.9% of eyes) in the 0.20-mg group did not require rescue therapy at 24 weeks, suggesting that both doses were equally successful in controlling ROP. VEGF plasma levels were not altered in either group, indicating a limited systemic drug exposure.35
Building on the evidence of CARE-ROP, the RAINBOW study was the first global, phase 3 randomized controlled trial on ranibizumab in ROP. A total of 225 patients from 87 centers were enrolled and randomized 1:1:1 to ranibizumab 0.2 mg, ranibizumab 0.1 mg, or laser.36 The primary outcome measure was survival with no active retinopathy, no unfavorable structural outcomes, and no need for a different treatment modality at or before 24 weeks.35
Treatment success occurred in 80% of infants receiving ranibizumab 0.2 mg compared with 75% of infants receiving ranibizumab 0.1 mg and 66% of infants after laser therapy (Figure 8). Statistical significance in the comparison of ranibizumab 0.2 mg versus laser therapy was narrowly missed (odds ratio 2.19 [95% CI 0.99-4.82]; P = .0507). “One might ask why this success rate was lower compared to CARE-ROP? First, the definition of treatment success in RAINBOW was quite strict, including the fact that to qualify, infants had to survive until the primary endpoint. In addition, they obviously could not have any active ROP at the primary endpoint and no treatment except study treatment, but they could also not have any unfavorable structural outcomes,” said Prof. Stahl. Unfavorable structural outcomes (defined as any one of retrolental membrane obscuring the view of the posterior pole; substantial temporal retinal vessel dragging causing abnormal structural features or macular ectopia; posterior retinal fold involving the macula; and retinal detachment involving the macula) were found more frequently in the laser arm than in the ranibizumab 0.2-mg arm. In terms of safety, death, serious and non-serious systemic adverse events and ocular adverse events were evenly distributed between groups. Plasma VEGF levels were measured at baseline and at around days 14 and 28 in each treatment group, and these showed no evidence of systemic suppression (Figure 9).36 The 5-year extension study of RAINBOW is currently ongoing, with results expected in 2022.36
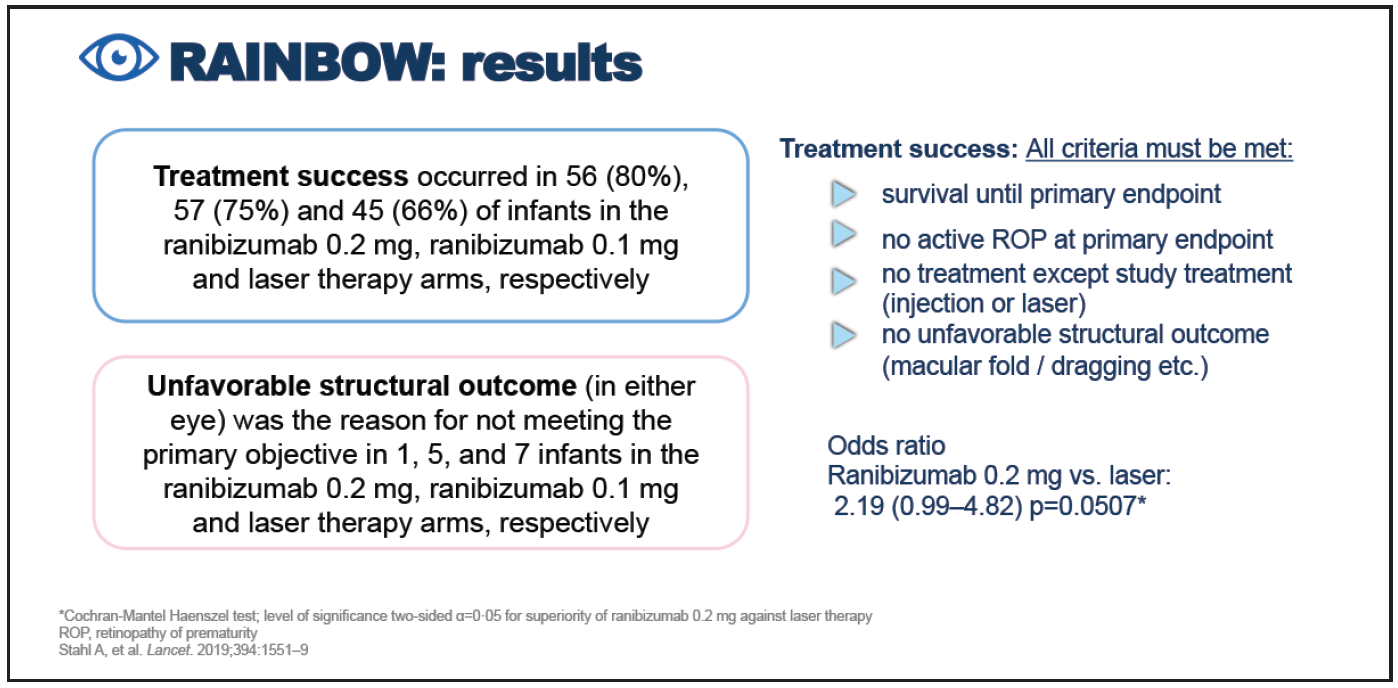
Figure 8. Results of the RAINBOW study.

Figure 9. Systemic VEGF levels in RAINBOW.
Based on the results of RAINBOW, ranibizumab was approved in the European Union in September 2019 for the treatment of ROP in preterm infants with Zone 1 (Stage 1+, 2+, 3 or 3+), Zone 2 (Stage 3+), or AP-ROP disease.37
Highlights
- Retinopathy of prematurity (ROP) registries provide important information on the real-world use of anti-VEGF therapy in ROP.
- In the RAINBOW study, a treatment success rate of 80% was achieved with ranibizumab 0.2 mg versus 66% with laser.
- Infants treated with ranibizumab 0.2 mg were twice as likely to achieve treatment success versus laser which was considered clinically relevant.
- Ranibizumab 0.2 mg was well tolerated in patients with ROP and the safety profile was as expected in a preterm population.
- In September 2019, ranibizumab was approved in the European Union for the treatment of ROP in preterm infants with Zone 1 (Stage 1+, 2+, 3 or 3+), Zone 2 (Stage 3+), or aggressive posterior-ROP disease.
