“We often fail to properly recognise how important the advent of anti-VEGF injections was to our patients,” said Prof. Peter Kaiser. Observational studies and theoretical modelling suggest that the incidence of blindness due to nAMD has reduced by between 47 and 72% since the introduction of anti-VEGF therapy (Figure 1).5-8
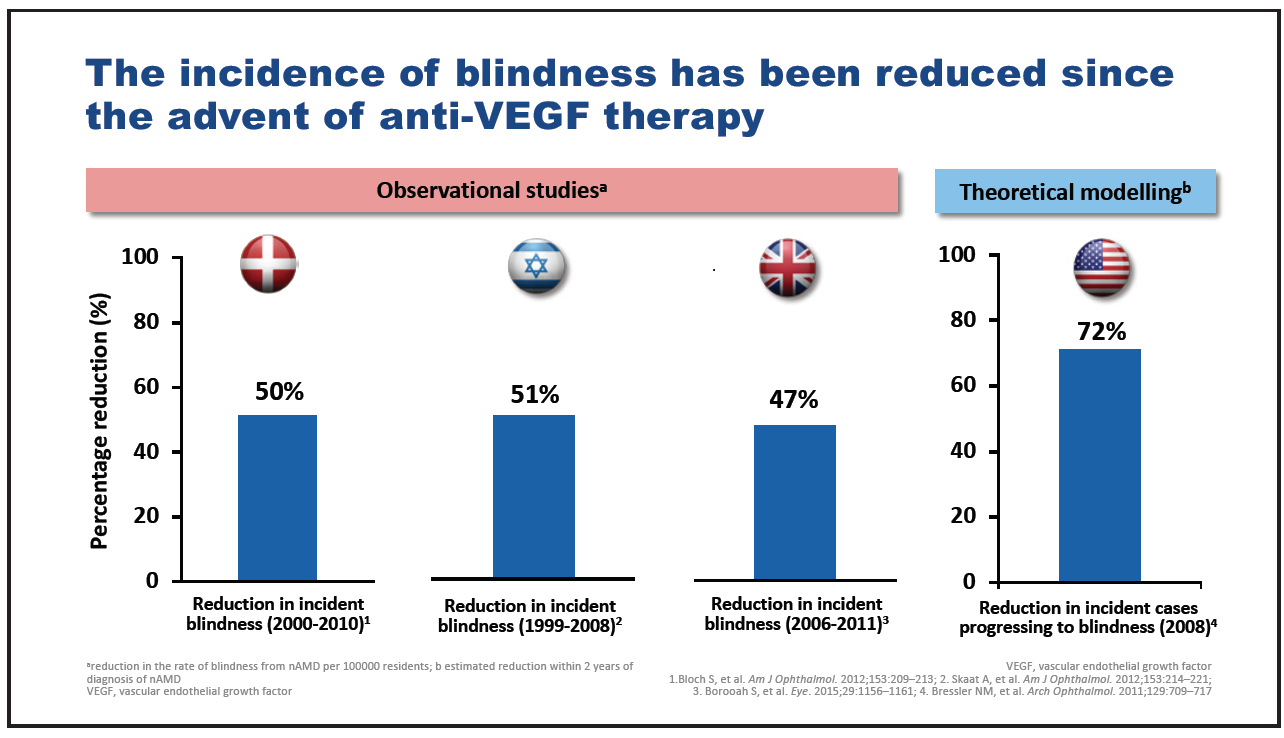
Figure 1. Incidence of blindness following introduction of anti-VEGF therapy.
However, while impressive visual gains are achieved with anti-VEGF therapies in clinical trials, the results reported in real-world studies are often comparatively disappointing. For example, in the ANCHOR randomized controlled study, patients exhibited a mean VA gain from baseline to month 12 of 11.3 letters,9 while, in contrast, patients assessed in the real-world LUMINOUS study gained an average of 3.1 letters over 12 months.10 This discrepancy is generally attributed to a difference in the average number of injections received by patients between the two settings. Patients in the ANCHOR study received a mean of 12 ranibizumab injections in the first year of treatment while those in LUMINOUS received just five injections.9,10 The challenge for retinal specialists is to provide effective treatment that maximizes and maintains visual gains without overburdening the patient with too-frequent clinic visits and injections. Treatment burden is widely recognized as an important issue in nAMD management: of 1,029 retinal specialists responding to the American Society of Retina Specialists Preferences and Trends survey in 2018, 73% of those from the United States and 66% of international respondents stated that reducing treatment burden was one of the greatest current unmet needs with regard to treatment for nAMD (Figure 2).11
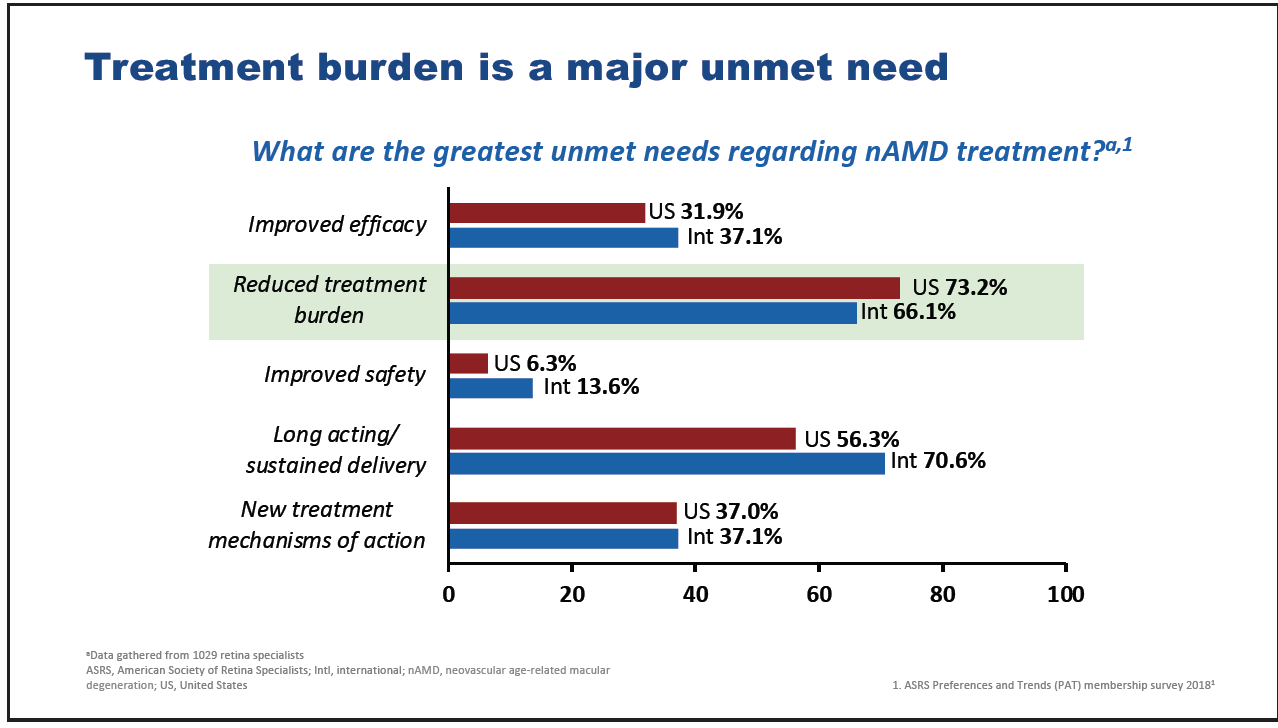
Figure 2. Major unmet needs in nAMD according to American Society of Retina Specialists members.
Consideration of retinal fluid plays an important part in the optimization of treatment and management of nAMD, at all stages from diagnosis to long-term maintenance therapy. Intraretinal and subretinal fluid visualized using OCT are important diagnostic criteria for nAMD.12 Timely diagnosis is important, as is a rapid response once nAMD is confirmed, since shorter times from diagnosis to first treatment are associated with better visual outcomes.13,14 “Studies repeatedly show that fellow eyes with nAMD have better outcomes than the first-treated eye,” said Prof. Kaiser. “The reason is that the fellow eye’s conversion to nAMD is often picked up early at a routine visit for the other eye. Reducing the time to first treatment as much as possible in order to similarly optimize outcomes, even in the first eye, is a priority for us from a health care standpoint.” During follow-up, the presence of any retinal fluid signifies a disease state which needs to be controlled with appropriate treatment strategies to prevent irreversible vision loss.15-17 Treatment guidelines from EURETINA,18 the Royal College of Ophthalmologists,19 and the American Academy of Ophthalmology20 recommend that retreatment decisions are based on the presence of fluid, predominantly subretinal, intraretinal, and subretinal pigment epithelium (RPE) fluid, as markers of disease activity (Figure 3). Control of fluid may also have an important role to play in the endeavour to reduce treatment burden in nAMD. The development of more potent therapies that control fluid in a more durable fashion compared with current anti-VEGF therapies should allow retinal specialists to optimize visual outcomes while at the same time reducing treatment burden.21,22

Figure 3. Guidelines for the treatment of nAMD.
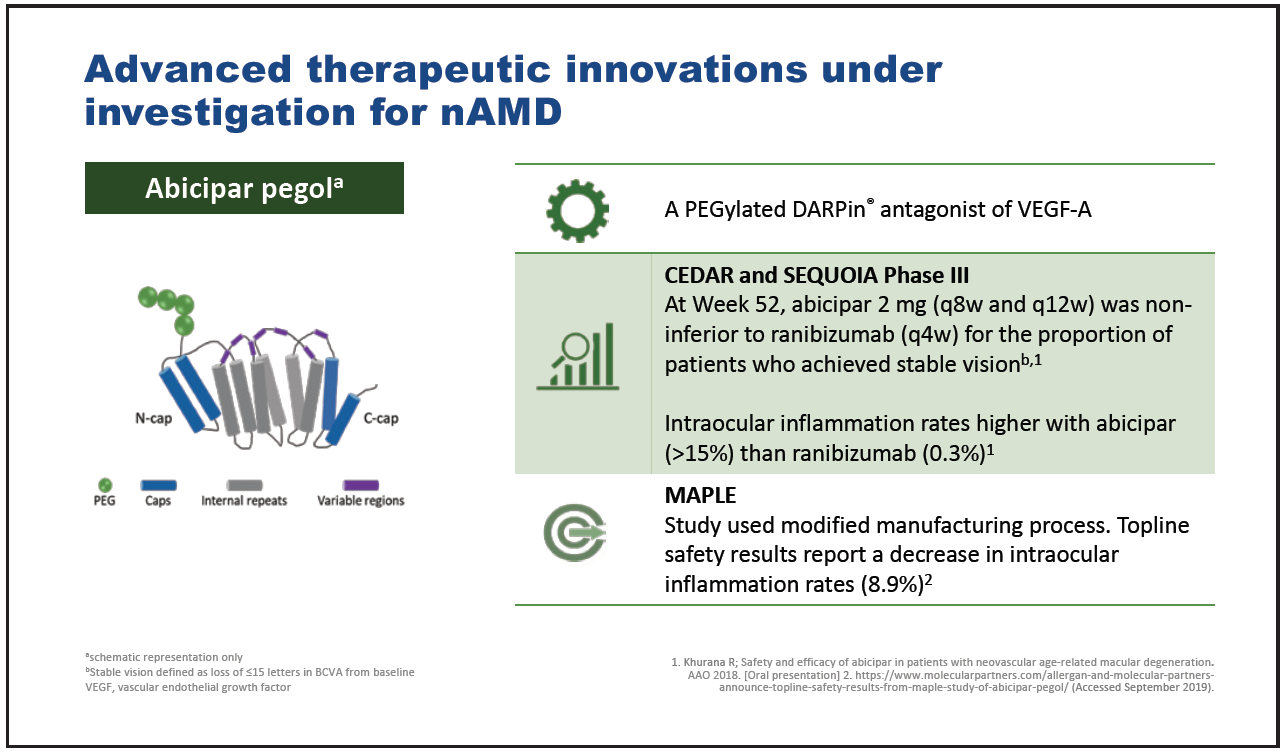
Figure 4. Abicipar pegol.
Three advanced therapeutic innovations currently under investigation for nAMD are abicipar pegol,23,24 faricimab,25-27 and brolucizumab.28 Abicipar pegol is a PEGylated DARPin antagonist of VEGF-A which in phase 3 clinical trials has demonstrated noninferiority to ranibizumab in terms of the proportion of patients achieving stable vision(Figure 4).23 Intraocular inflammation rates were markedly greater with abicipar pegol than with ranibizumab, though subsequent modification of the abicipar pegol manufacturing process has reduced these significantly.23,24
Faricimab is a novel bispecific antibody targeting VEGF-A and angiopoietin-2 which, in a phase 2 trial, demonstrated similar VA gains when given every 12 or 16 weeks to ranibizumab given every 4 weeks (Figure 5).25 Further studies comparing faricimab with aflibercept are ongoing.26,27
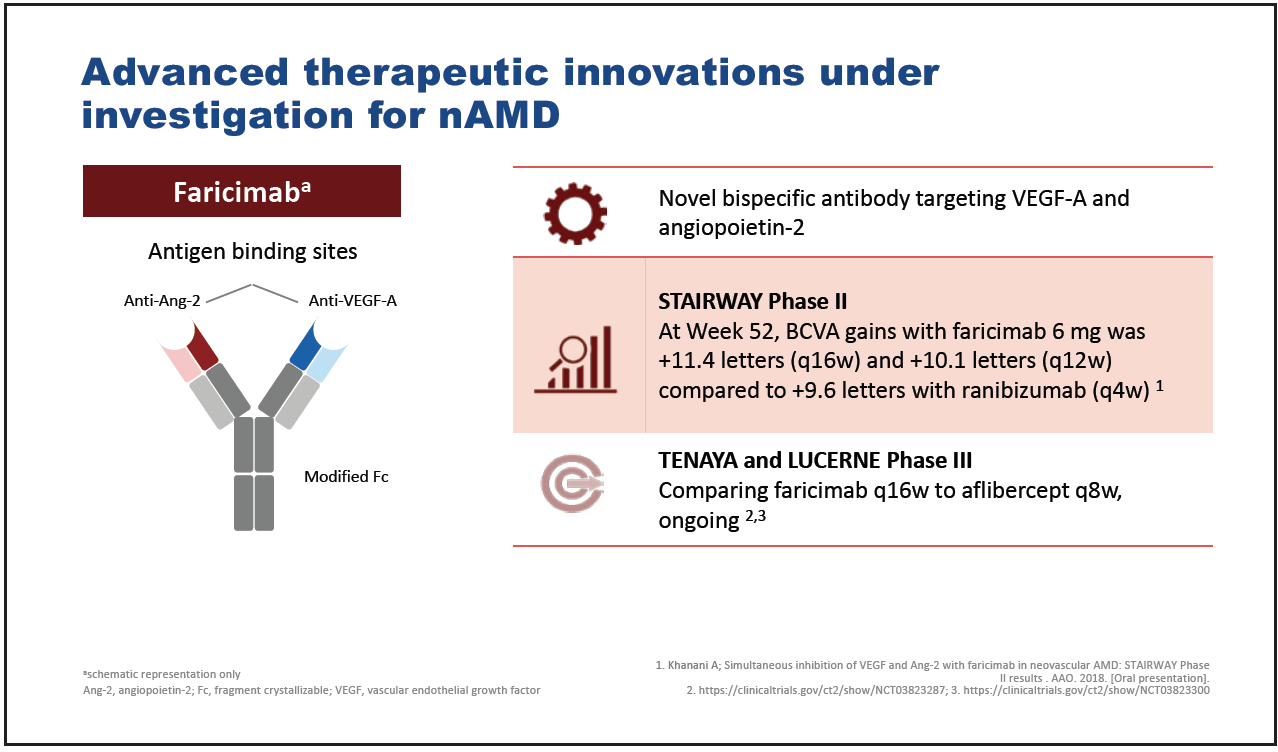
Figure 5. Faricimab.
Brolucizumab is a small, single-chain antibody fragment that inhibits VEGF-A and which was designed specifically for ophthalmic use (Figure 6).28 Based on the results of the phase 3 studies HAWK and HARRIER, brolucizumab was approved by the US FDA on October 8, 2019, for the treatment of nAMD29 and has since received approval in the EU, Canada, Australia, Japan, UAE, and Albania.
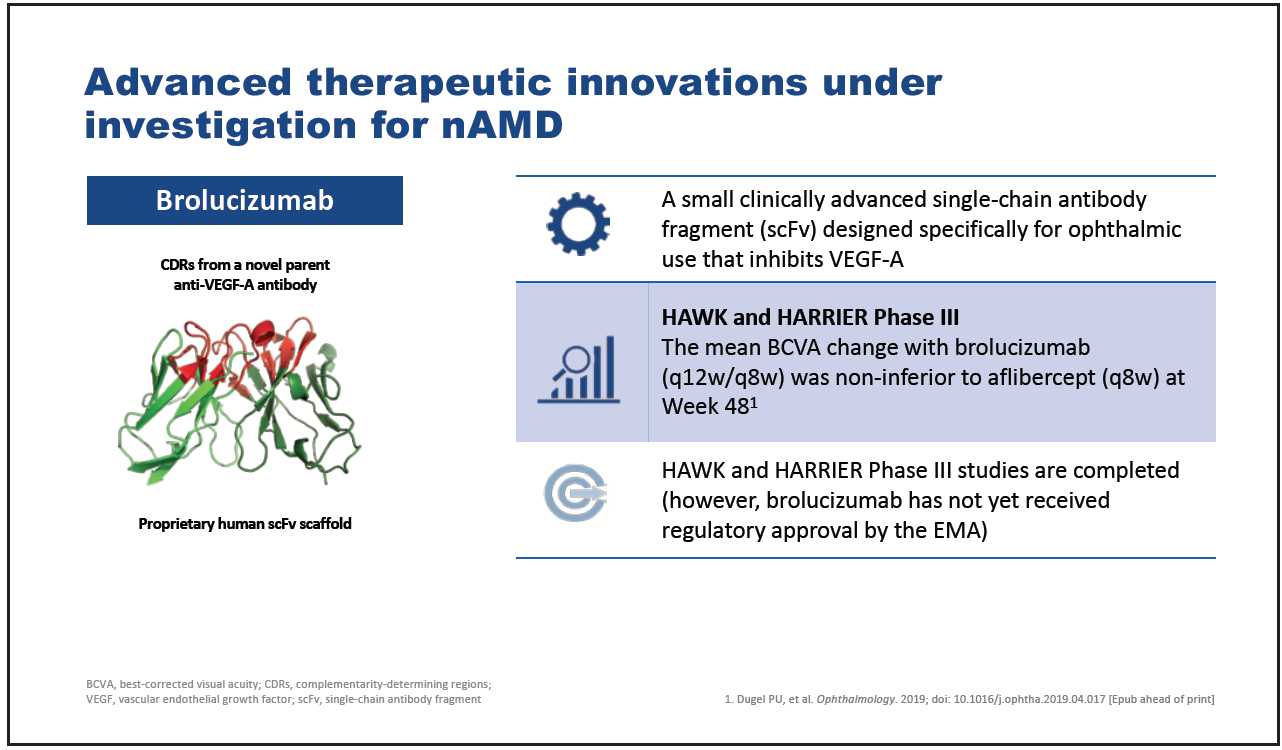
Figure 6. Brolucizumab.
To summarize, reduced treatment burden is a major unmet need in nAMD. Fluid in the retina is indicative of disease activity and needs to be controlled with appropriate treatment strategies to prevent irreversible vision loss. More potent, durable therapies to control fluid should optimize visual outcomes at the same time as reducing treatment burden. “We have three drugs on the horizon which will hopefully meet these criteria: being potent, decreasing fluid, and, most importantly, reducing treatment burden,” said Prof. Kaiser.
Highlights
- Reduction of treatment burden is an important unmet need in nAMD.
- Fluid in the retina is an indicator of disease activity which must be appropriately controlled in order to prevent irreversible vision loss.
- More potent and durable therapies to control fluid should optimize visual outcomes and reduce treatment burden in nAMD.
