Dr. Wykoff: How are you using imaging for patients with DR?
Dr. Baumal: I use imaging on a discretionary basis. If there is moderate NPDR or if there is any suspicion of DME, I will certainly order an OCT. However, I do not image patients with mild NPDR, primarily because the most relevant findings are identified on clinical examination. Also, some individuals may not have insurance coverage or else do not want to deal with copays and deductibles.
Dr. Coney: The severity of the DR is the most important factor in how I use imaging. For the patient with NPDR without macular edema (ME), my preference is to use the first patient encounter to focus on education and to establish the follow-up timeline and then to obtain a fluorescein angiogram (FA) on the second visit. As we follow patients over time, we can refer back to the FA to get a sense of whether there are any relevant changes to the microvasculature. Furthermore, showing patients serial FA images can help reinforce education about modifiable risk factors and lifestyle changes that will help them to gain better control of their diabetes. In a similar vein, I also send FA images to the patient’s primary care doctor so that he or she is aware of any changes in the ocular health status.
Dr. Wykoff: In most cases, I obtain an OCT. Beyond that, I use my clinical exam to guide the use of additional imaging as necessary. For example, if a patient has normal OCT and a few microaneurysms, I typically do not obtain any additional imaging. In comparison, when there are scattered dot blot hemorrhages, I obtain a photograph and have a low threshold to order a wide-field angiogram.
Dr. Kiss: Unless there is an obvious reason not to, it is helpful to obtain an FA on every patient with a high HbA1c (I tend to use 10) to at least establish a baseline, but also to have something to refer back to as I follow the patient over time. Ideally we are getting a photograph and an OCT image for all patients, but we may not bill for it if there is no pathology present.
Dr. Baumal: My preference is to start with the clinical examination and then to perform directed imaging with OCT, color photography, and/or FA. I typically perform FA when the results are going to direct treatment. For instance, in a patient with PDR, the amount and extent of leakage may affect how often I administer anti-VEGF injections. Patients with DME, especially if they have not responded to therapy, are another category of patients in whom FA is warranted. Also, FA may identify foveal or peripheral ischemia, which may direct the clinician’s therapeutic options. The reason I am cautious with FA is that it is not a completely benign test. I have seen a patient experience a severe anaphylactic reaction to fluorescein dye, which fortunately resolved with epinephrine.
Dr. Chiang: I have become more of a minimalist and am selective about using FA because of the safety issues just mentioned and because it often does not alter my management plan in NPDR cases. These days we can often arrive at a conclusion to initiate anti-VEGF therapy for DME based on a good clinical exam and OCT alone. I will obtain a fundus photograph for documentation purposes, but mostly I reserve FA to evaluate suboptimal treatment response, for preoperative assessment of ischemia, and to guide laser treatment.
Dr. Kiss: FA may not impact treatment, per se, but the results can suggest a need for closer monitoring. There are a couple pieces of evidence that may be important.
- In a prospective series of patients, Lloyd Clark, MD, showed that a pattern of predominantly peripheral lesions was associated with a 100% chance of progressing to vision-threatening disease over 4 years.14
- That pattern may be missed on clinical exam, and even the classic ETDRS seven-field imaging may miss it; this was confirmed in the DRCR.net Protocol AA study.15
- The main goal of the study was to compare the level of concordance in grading DR severity using ETDRS seven-field imaging and ultrawide-field imaging, as well as to identify patterns of pathology that are indicative of higher risk of progression.
- The study found that standard ETDRS seven-field missed pathology in the far periphery in about 10% of cases.
- That leads me to believe that clinical examination alone may be insufficient and causes me to have a low threshold for getting an FA, particularly if the patient’s HbA1c is not properly controlled or if other risk factors are present.
- The FA may not change whether or when I inject, but it does affect how frequently we follow-up.
- It may also give a sense of how often we will need to inject anti-VEGF agents. It is certainly not exact, but the patient with DME with elements of proliferative disease detected on FA is going to be followed differently than the patient with DME, 20/40 VA, and no ischemia or nonperfusion on FA.
Dr. Wykoff: I typically consider an FA when there is anything beyond background retinopathy, and my preference is to do that before starting treatment. My rationale is that if there is any proliferative disease, it will be masked by anti-VEGF injections. Proliferative disease is a marker for a higher risk eye; it is important to identify this and adjust the management plan accordingly—inclusive of when injections are administered and how often I follow the patient. If there is a concern about the patient’s ability to be compliant with the follow-up schedule, I will consider panretinal photocoagulation (PRP).
Case 2: Considerations for Initiating Therapy With Anti-VEGF Agents
By Szilárd Kiss, MD
Case Overview:
- A 65-year-old man presents for a second opinion for a 3-week history of floaters and decreased vision in the right eye.
- Type 2 diabetes with hemoglobin A1c (HbA1c) 9.7%.
- Medical history: hypertension, hypercholesterolemia, and slightly elevated prostate-specific antigen levels.
- Diagnosed with posterior subcapsular cataracts 3 years prior.
- Never received any treatment in either eye.
- Diagnosis: proliferative diabetic retinopathy in each eye with neovascularization and large areas of ischemia, no macular edema; imaging of left eye demonstrates vitreous hemorrhage (Figure 1).
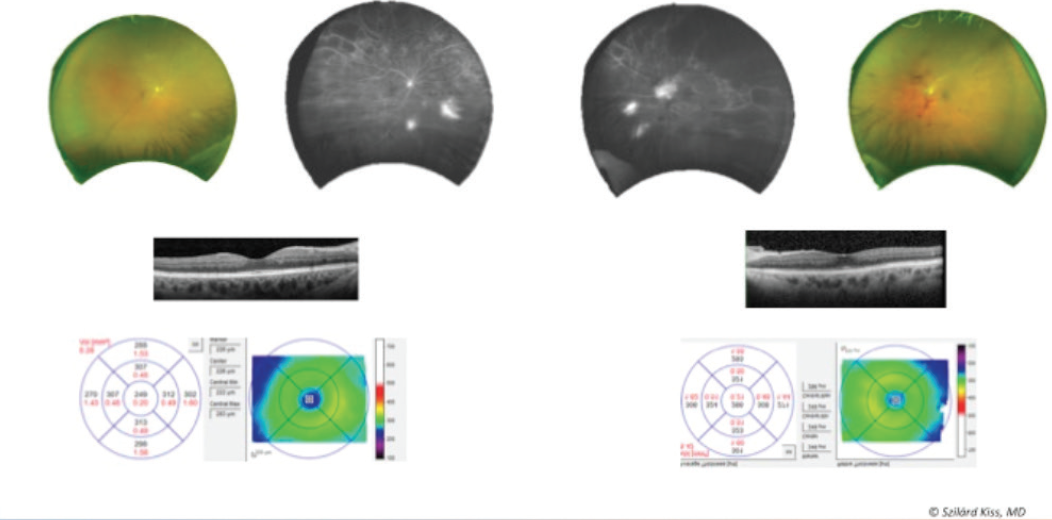
Figure 1. Vitreous hemorrhage OS.
Treatment Overview:
- After three ranibizumab (Lucentis, Genentech) injections, fluorescein angiogram (FA) showed resolution of vitreous hemorrhage and improvement but not resolution of the neovascularization (Figure 2).
- After six more injections of ranibizumab (nine total) the patient returned 3 days prior to scheduled cataract surgery in the left eye; imaging demonstrated worsening of neovascularization in the right eye (Figure 3).
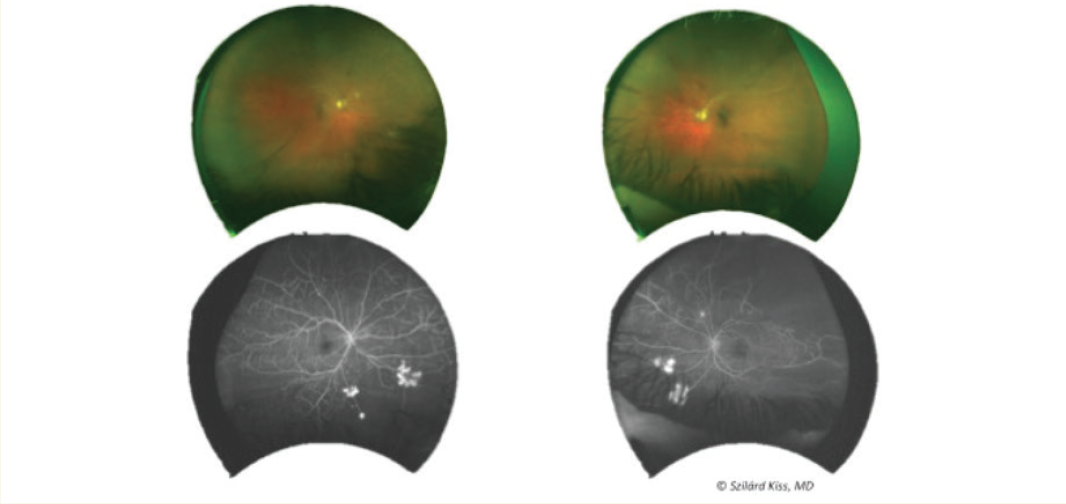
Figure 2. FA showed resolution of vitreous hemorrhage and improvement but no resolution of neovascularization after three ranibizumab injections.
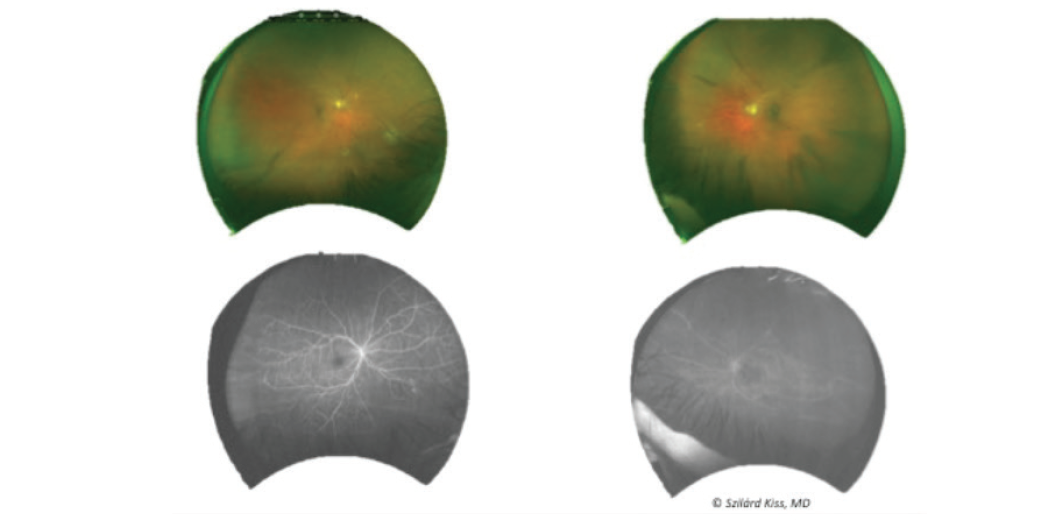
Figure 3. FA showed worsening of neovascularization after nine total ranibizumab injections OD.
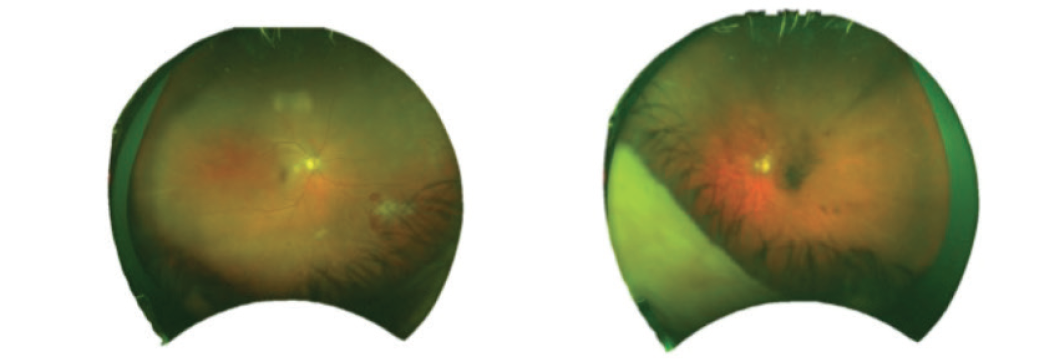
Figure 4. Wide-field imaging demonstrated apparent worsening of neovascularization OS.
Dr. Kiss: Is anyone using OCT angiography (OCT-A)?
Dr. Baumal: I use OCT-A to image most, if not all, diabetic eyes. In addition to the en face OCT-A depth resolved image of the fovea circulation, a coregistered structural OCT is also produced. The images of the fovea can reveal an enlarged foveal avascular zone, capillary dropout, microaneurysms, and intraretinal cysts. Currently US FDA approved OCT-A devices produced 3 x 3-mm and 6 x 6-mm images. Our center at New England Eye Center has access to an OCT-A device that is not commercially available at this time, which produces 12 x 12-mm images and also a mosaic of five 12 x 12-mm OCT-A images, essentially producing an ultrawide-field OCT-A. These images can be obtained noninvasively in under 5 minutes without the use of fluorescein dye and can demonstrate peripheral retinal neovascularization, intraretinal microvascular abnormalities, or ischemia. Peripheral pathology can similarly be imaged with the commercial devices by scanning the smaller scan sizes directed eccentrically. From my experience, OCT-A has enormous potential for noninvasive screening and to direct therapy to high-risk diabetic eyes; this technology will only continue to improve and play a larger role.
Dr. Wykoff: I have access to OCT-A in most of my clinics, and the images in DR are impressive. At this point, however, I do not consider OCT-A essential to the management of DR. Over time I believe we will see incorporation of OCT-A algorithms into DR management approaches, and I am excited about the ability to quantify area of retinal nonperfusion longitudinally in a way not possible with liquid-based angiography.
For the patient with NPDR who is being followed off treatment, how often do you repeat imaging?
Dr. Chiang: I repeat imaging studies, such as FA, only if I see changes on the clinical examination, which is still the centerpiece of their office visit. I usually have their baseline and/or latest wide-field fundus photograph up on the screen for direct comparison. Even if the imaging is covered by insurance, many patients have high deductibles, and I try to factor in their economic cost whenever possible.
Dr. Coney: I agree. Unless there is a definitive reason, I do not repeat the FA. For OCT, if the baseline imaging was normal, and if there is good foveal contour and reflex with no structural abnormalities on a subsequent examination, there is no reason to repeat.
Dr. Baumal: The quality of fundus photography has improved to a point where we can be much more discerning about additional imaging. The clarity and what we are able to detect is such that we can easily refer back to previous images during a clinical examination to see if anything has changed. From a larger perspective, it is worth keeping in mind that the increasing prevalence of diabetes is going to mean more patients coming in with DR. Refining our imaging protocols so that we accomplish more targeted screening is going to help us be more efficient and streamlined as we deal with that larger volume.
Cases 3 and 4: A Learning Experience and Applying the Lessons Learned
By Joseph M. Coney, MD
Case 3 Overview:
- A 43-year-old white woman diagnosed with diabetes mellitus in 2008.
- Past medical history included uncontrolled diabetes for 9 years with hemoglobin A1c (HbA1c) 14%; hypertension; obstructive sleep apnea; peripheral vascular disease (i.e., previous partial toe amputation); two previous C-sections; and a smoking history.
- Medications: lisinopril, metoprolol, and insulin.
- VA is 20/25-1 OD and 20/20-1 OS.
- She has proliferative diabetic retinopathy (PDR) in both eyes with neovascularization.
Clinical Course:
- Anti-VEGF therapy was considered but not pursued.
- Panretinal photocoagulation (PRP) was performed based on the presence of neovascularization in the supratemporal region and below the disc (Figure 1).
- The patient did not comply with follow-up and returned 9 months later with advanced disease (Figure 2).
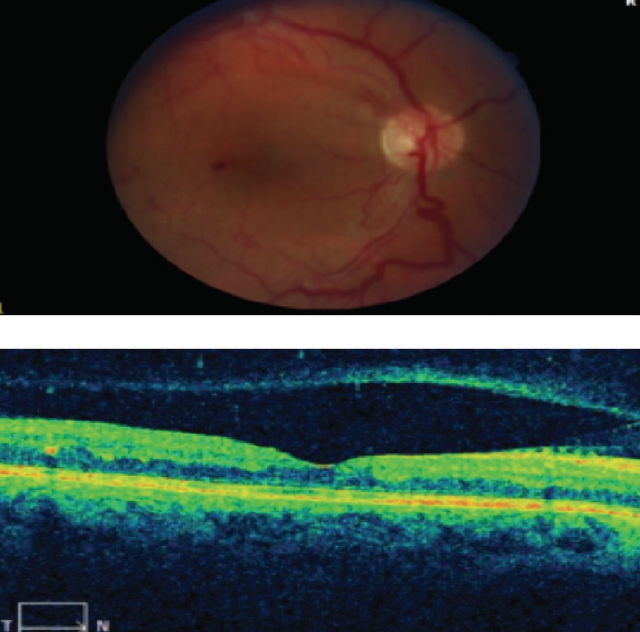
Figure 1. A presence of neovascularization in the supratemporal region and below the disc was shown.
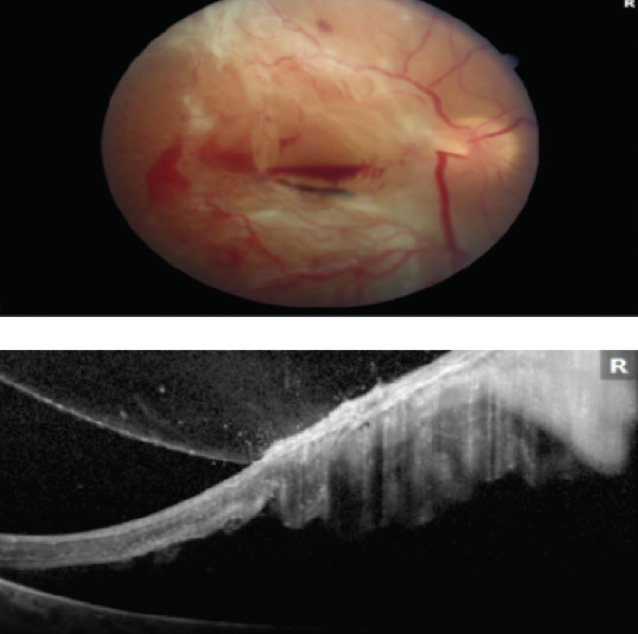
Figure 2. The patient presented with advanced disease 9 months after initial visit.
Case 4 Overview:
- A 49-year-old white woman with type 2 diabetes mellitus diagnosed on March 20, 2018.
- Past medical history: uncontrolled diabetes mellitus (HbA1c 13%); hypertension; peripheral vascular disease (s/p foot ulcer); and thyroid disease.
- Medications: levothyroxine, amlodipine, pantoprazole, and metformin.
- VA is 20/30-2 OU.
- Blood pressure is 117/64.
- Fluorescein angiogram (FA) showed capillary dropout in the periphery and all four quadrants OD, as well as venous beading (VB) (Figure 3A). FA of the left eye demonstrated VB and small areas of neovascularization in the periphery (Figure 3B).
- Diagnosis: severe nonproliferative diabetic retinopathy (NPDR) OD and early PDR OS.
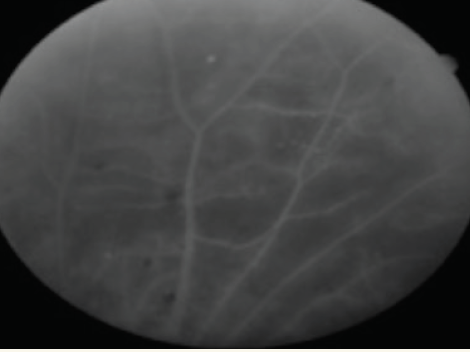
Figure 3A. Capillary dropout in all four quadrants and VB OD.
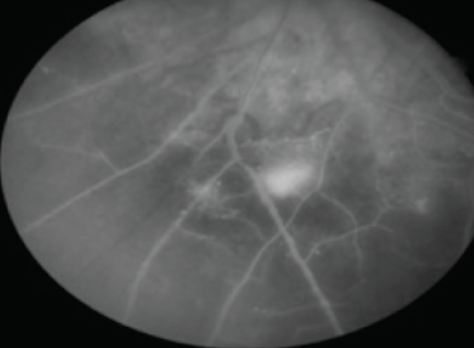
Figure 3B. Neovascularization elsewhere and nonperfusion with VB and extensive capillary dropout OS.
Clinical Course:
- A decision was made to use anti-VEGF therapy.
- At a follow-up visit after four monthly injections there was significant reduction in nonperfusion in each eye and VB had resolved OU (Figure 4A and 4B).
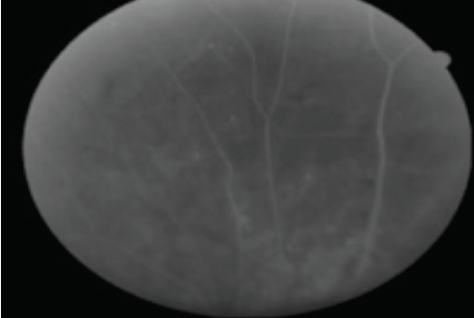
Figure 4A. Nonperfusion and resolution of VB OD.
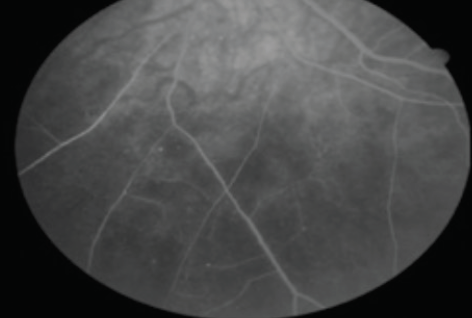
Figure 4B. Neovascularization elsewhere and nonperfusion with resolution of VB OS.
Discussion
Dr. Coney: In the first case presented here, the patient was ultimately treated with surgery, and although we stabilized the eye, the final VA was hand motions. These kinds of outcomes should be avoidable with the appropriate use of anti-VEGF therapy. I plan to continue for at least two more monthly injections, at which time I will repeat the FA to check the status of the neovascularization. In a patient like this, what is the best treatment interval after the loading dose?
Charles C. Wykoff, MD, PhD: There is a trend in diabetic retinopathy (DR) to use prn dosing, but the logic behind episodic therapy in DR does not sit well with me. I would treat this eye quarterly or even every 16 weeks if there is stability.
Allen Chiang, MD: It is interesting that the first case you presented deals with a patient who was noncompliant after laser. A prn treatment protocol really hinges on compliance: you cannot treat the patient who is progressing when he or she does not show up. And so, I agree and also hesitate on using prn treatment.
Szilárd Kiss, MD: I am becoming more convinced of the need for regular treatments regardless of whether there is neovascularization. The classic thinking in DR is that diabetes “burns itself out,” but we may be doing patients a disservice by using episodic anti-VEGF therapy and allowing it to progress in between.
Dr. Coney: In this patient, my impression was that the areas of nonperfusion on the baseline FA represented dead tissue. However, they appeared to improve over time.
Dr. Kiss: If we think about what anti-VEGF is used for in other areas of medicine, it is actually to normalize the vasculature, and that is what we do as well. Even in treating cancer, anti-VEGF is not used to starve the tissue of vascularization. Instead, it is used to normalize the vasculature so that the chemotherapy will be effective. DR is a vasculopathy, and thus with anti-VEGF therapy we are normalizing the vasculature, even in cases that are angiographically silent.
