To watch the presentation by Dr. Mark E. Pennesi at EURETINA, please click on the link at the end of this article.

“We are entering an age of gene therapy, with multiple trials of gene therapies for IRDs ongoing,” said Dr. Mark E. Pennesi. “At the same time, we are seeing a shift away from phenotypic diagnosis, such as rod-cone or cone-rod dystrophy, to defining these diseases by the gene that is involved.”
RPE65 mutation-associated retinopathy is a rare autosomal recessive IRD, which usually presents very early (often before the age of 5 years), with vision loss, nystagmus, and profound nyctalopia. Patients often show pigmentary degeneration and are myopic, although many will have preserved central vision at presentation.1,2 The natural history of the disease is for early, profound changes in VA, which worsen with age, with progressive loss of functional retina over time.3 “What is really important to understand about this disease is that it’s a progressive degeneration. By age 20, most patients are going to go legally blind and eventually they will go completely blind,” said Dr. Pennesi.
In 2017, voretigene neparvovec became the first gene therapy to be approved by the US FDA for an IRD.4 This was followed by EMA approval in 2018.5 In January 2018, Novartis entered into a licensing and supply agreement with Spark Therapeutics to develop, register, and commercialize voretigene neparvovec outside the United States. Spark retains the US rights under the label voretigene neparvovec-ryzl.
Voretigene neparvovec is a recombinant adeno-associated virus serotype (AAV) 2 vector used to introduce a functional copy of the RPE65 gene into the retinal pigment epithelium (RPE) cells of patients with confirmed bi-allelic RPE65 mutation-associated retinal dystrophy. Treatment with voretigene neparvovec drives expression of cDNA encoding human RPE65 kDa (RPE65) protein within the RPE cells.
Voretigene neparvovec is given as a subretinal injection. A dose of 0.3 mL containing 1.5 x 1011 vector genomes is injected beneath the retina using a fine-gauge cannula to form a bleb, which is typically resorbed within 24 hours of surgery. The location of the bleb defines the treatment area, as the treatment effect typically doesn’t spread beyond the bleb, so the injection is typically directed toward the macula, although not in the immediate vicinity of the fovea.
The approval of voretigene neparvovec was based on the results of a randomized, controlled, open-label, phase 3 trial.6 Individuals aged 3 years or older with a confirmed genetic diagnosis of bi-allelic RPE65 mutation were randomly assigned (2:1) to treatment (voretigene neparvovec; n = 21) or control (no treatment; n = 10) (Figure 1). The primary efficacy endpoint was the change in bilateral multiluminance mobility test (MLMT) performance (change in lux score for the lowest passing light level) at 1 year relative to baseline. “The MLMT is like a maze with obstacles that the patient walks through. It’s performed at various light levels from 400 lux (equivalent to a bright office environment) down to 1 lux (equivalent to a moonlit night). The score that a patient achieves is based on the light level at which they can successfully complete the maze,” said Dr. Pennesi (Figure 2).7 At 1 year, mean bilateral MLMT score improved by 1.8 light levels in the treatment group versus 0.2 levels in the control group (P = .0013).6 It was found that 65% of participants who received treatment passed the MLMT at the lowest luminance level tested.
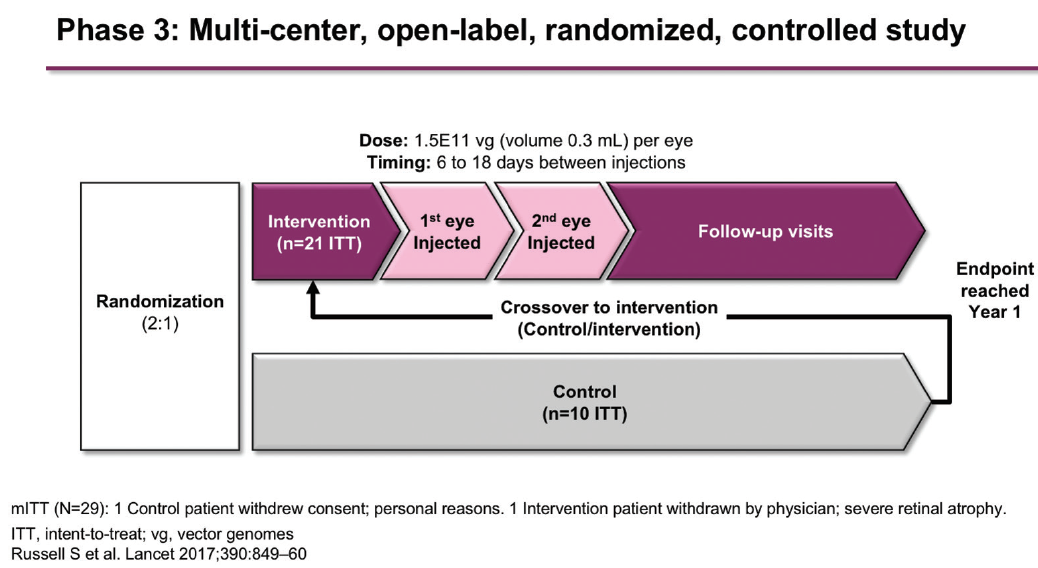
Figure 1. Design of phase 3 study of voretigene neparvovec.
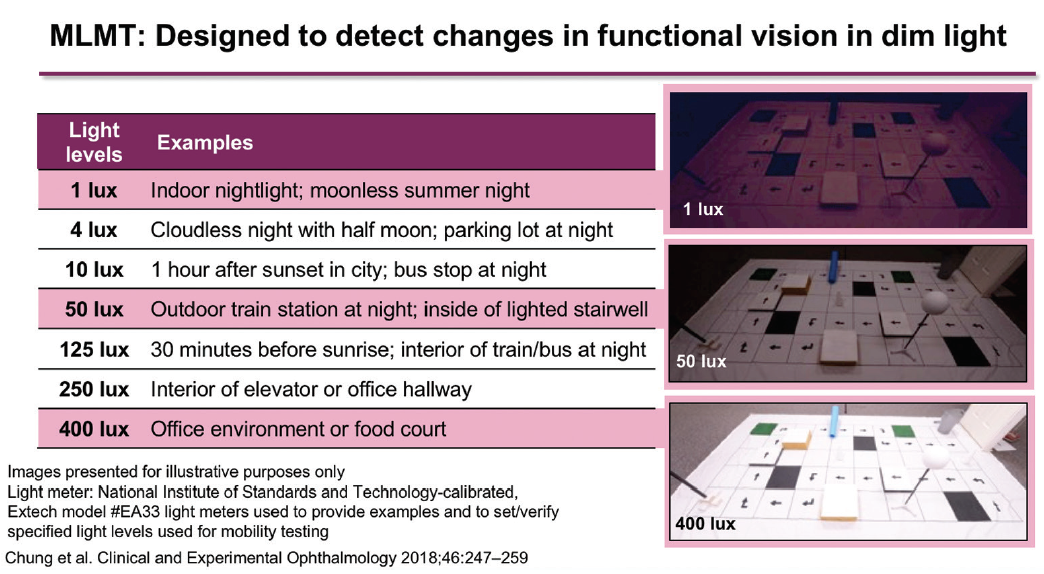
Figure 2. The MLMT.
Secondary endpoints included full-field stimulus threshold (FST) testing (averaged over both eyes) and BCVA (averaged over both eyes). Patients in the treatment group experienced a rapid improvement by day 30, which remained stable at 1 year, while the control group showed no change (P = .0004). BCVA improved by a mean of 8.1 letters in the treatment group versus 1.6 letters in the control group, but this difference was not statistically significant.6
These results were sustained to 4 years, with a mean change in bilateral MLMT score of 1.7 levels in the treatment group (Figure 3).8,9 Patients who were initially randomized to the control arm were eligible to receive treatment at 1 year. These patients achieved a mean change in bilateral MLMT score of 2.4 levels at 4 years (3 years after treatment). Similar sustained results in the treatment group and improvements in the control group following crossover were seen on the secondary endpoints of FST (Figure 4) and BCVA.8
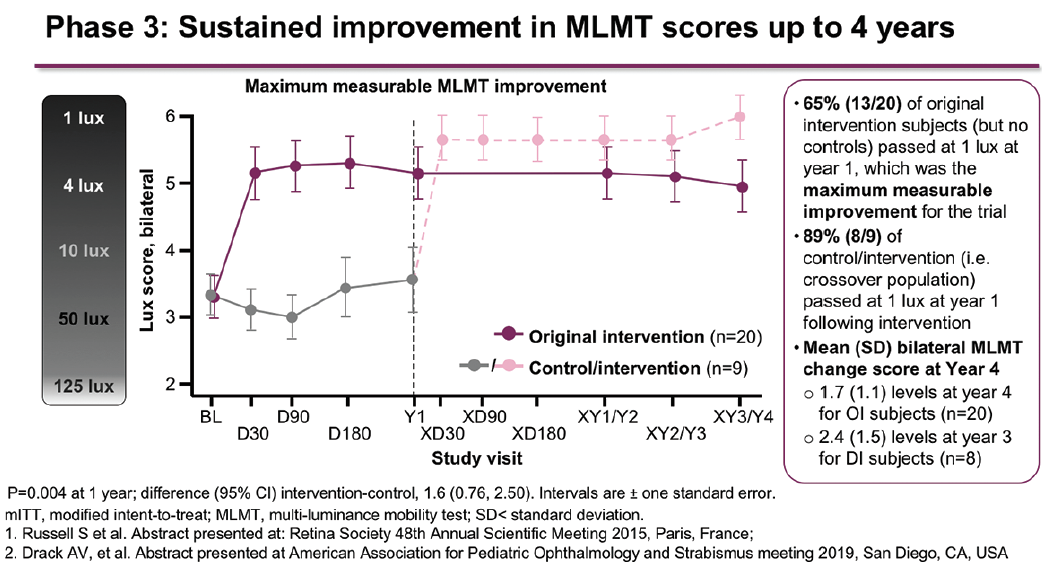
Figure 3. Mean change in bilateral MLMT scores up to 4 years in the phase 3 study.
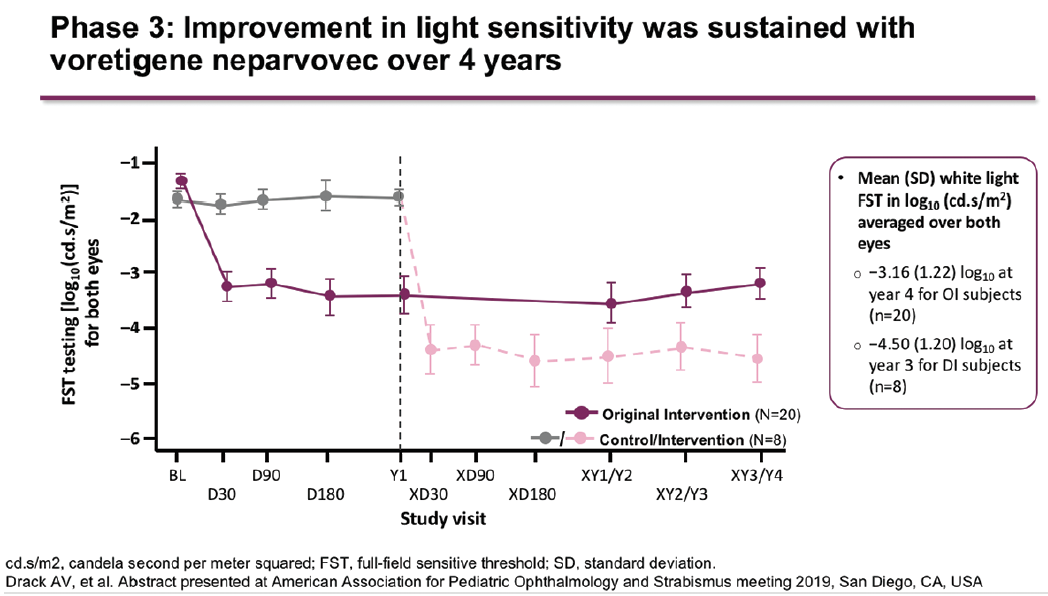
Figure 4. Change in light sensitivity up to 4 years in the phase 3 study.
“At the Casey Eye Institute, we have treated six patients with voretigene neparvovec as of August 2019,” said Dr. Pennesi. “Of these, five were bilateral cases, with the patient ages ranging from 4 to 33 years.” One example case is a 13-year-old male, who was diagnosed as a child with early-onset severe retinal dystrophy and in whom later genetic testing confirmed bi-allelic RPE65 mutations. Before surgery, the patient presented with BCVA of 20/400 in both eyes. Three months after treatment with voretigene neparvovec in both eyes, his VA had improved to 20/150 in the right eye and 20/200 in the left eye. FST with blue stimulus (more selective for rods) showed a one-log fold improvement in sensitivity, while FST with red stimulus (more selective for cones) showed an approximate half-log improvement. Similar results were seen with dark-adapted perimetry, which revealed a profound increase in sensitivity to blue light and a smaller, but still notable, improvement in sensitivity to red light. Improvements occurred in the approximate area that injection had taken place.
To watch the presentation by Dr. Mark E. Pennesi at EURETINA 2019, please click here
