To watch the presentation by Dr. José-Alain Sahel at EURETINA, please click on the link at the end of this article.

Following the approval of voretigene neparvovec for RPE65-related retinal dystrophy, gene therapy for IRDs is becoming a reality. “However, it’s very important to realize that you cannot start treatment from scratch,” said Dr. José-Alain Sahel. “Patients eligible for this treatment have been identified throughout many years of work, identification of the gene, identification of a pattern in the family history, and very deep phenotyping to identify how many cells are still viable and the status of the retina. Treatment centers must be able to handle that, and they must also have very skilled surgeons. Even with the best gene therapy, the result may be compromised if surgery is not performed as well as it can possibly be.”
Treatment centers for voretigene neparvovec must fulfill a number of risk management plan criteria. The presence of an ophthalmologist with expertise in care and treatment of patients with IRDs is required, as is the presence of, or affiliation with, a retinal surgeon experienced in subretinal surgery and capable of administrating voretigene neparvovec. Finally, there must be a clinical pharmacy capable of handling and preparing AAV vector-based gene therapy products.21
In one such expert center, Centre Hospitalier National d’Ophtalmologie des Quinze-Vingts in Paris, treatment with voretigene neparvovec has been ongoing since December 2018 under a temporary authorization of use. Up until September 2019, nine patients have been treated with the gene therapy. Surgical posterior vitreous detachment, subretinal injection, and air fluid exchange were performed according to the recommended surgical procedure (Figures 8 and 9). Oral prednisolone was given before and after surgery. Topical postoperative treatment was the same as any retinal surgery.
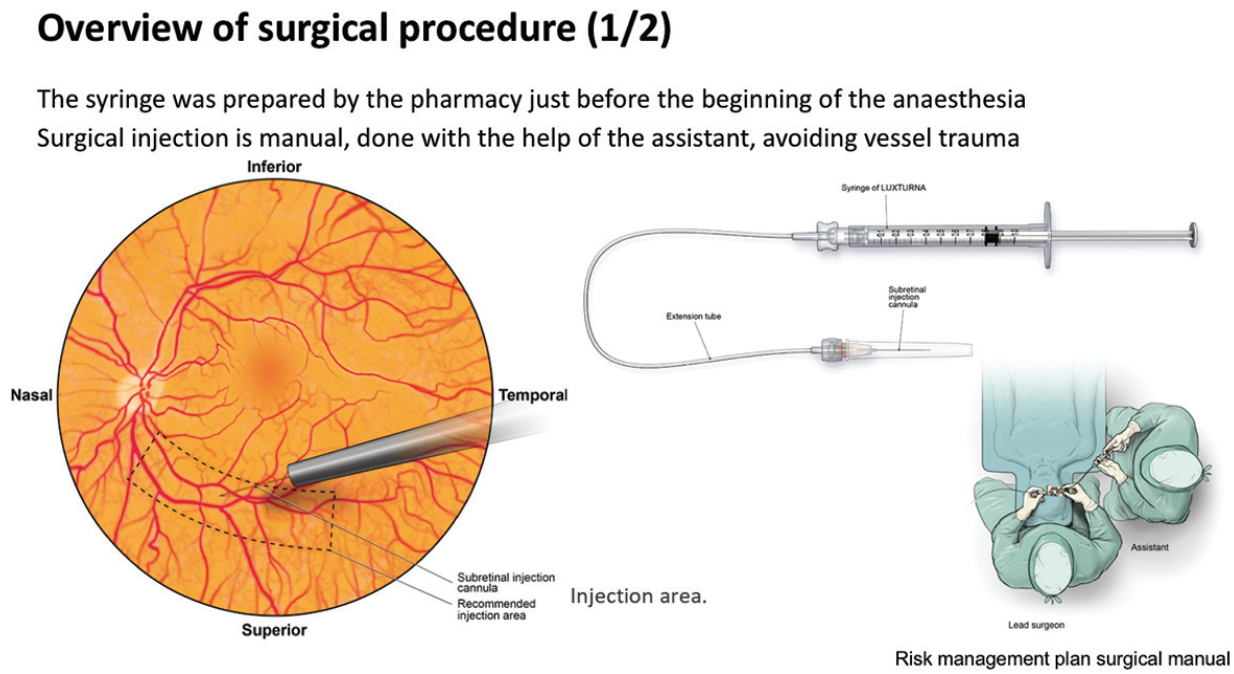
Figure 8. Surgical procedure (one of two).
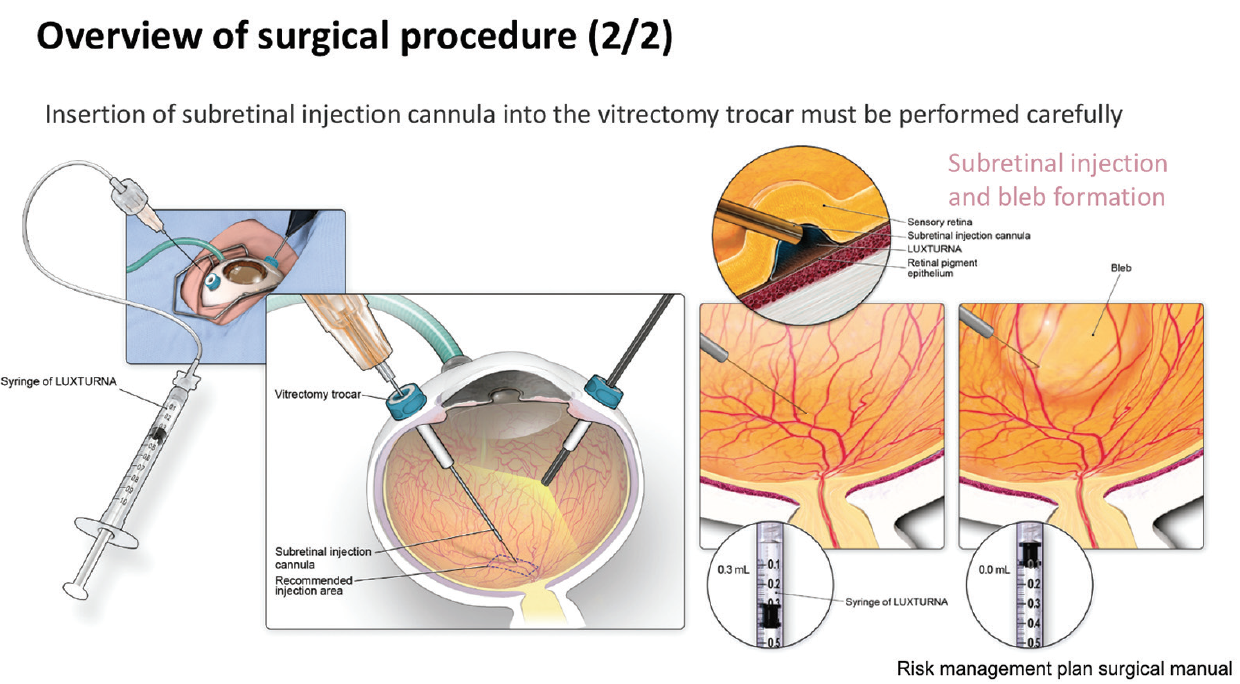
Figure 9. Surgical procedure (two of two).
“Key aspects to note in this procedure are that placement of the injection cannula has to be extremely careful to avoid any damage to the macula,” said Dr. Sahel. “Also, we have experienced a level of unpredictability as to where the bleb is going to form. The surgeon has to make sure that the bleb eventually reaches the macula. Very careful fluid exchange can ensure that the bleb progressively moves beneath the macula, but it mustn’t be forced as this risks damaging the photoreceptors.”
The first two patients treated with voretigene neparvovec at Centre Hospitalier National d’Ophtalmologie des Quinze-Vingts were sisters who presented with Leber congenital amaurosis at the age of 10 (sister A) and 8 (sister B) years. Both sisters had bi-allelic mutations of RPE65 causing low vision, nystagmus, photophobia, hemeralopia, and night blindness. The sisters received the treatment between December 2018 and February 2019 in both eyes with a 1-week interval between eyes.
Sister A had baseline VA of 20/200 in the right eye and 20/320 in the left eye. At 6 months after treatment, this had improved to 20/125 in the right eye and 20/250 in the left eye. Goldmann visual field testing showed some improvement, while FST results showed dramatic improvements from around -4 dB at baseline in both eyes to approximately -31 dB at 6 months (Figure 10).
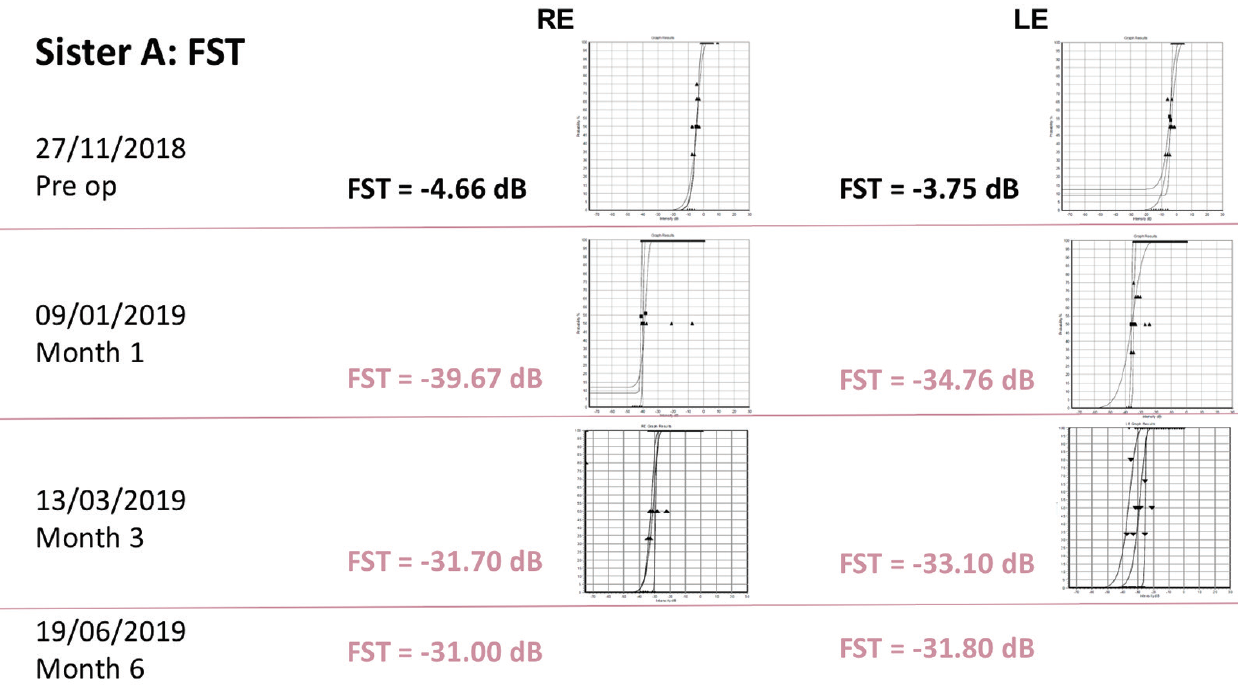
Figure 10. FST results in sister A.
Like her sibling, sister B had baseline VA of 20/200 in the right eye and 20/320 in the left eye. At 3 months after treatment, this had improved to 20/125 in the right eye and remained stable in the left eye. Improvements were again seen on Goldmann visual field testing and FST testing.
Since functional improvements achieved with voretigene neparvovec are not fully reflected by changes in BCVA, outcome measures are required that can quantify improvements in dark-adapted vision. “We have developed a platform called Streetlab, which allows us to monitor in detail the impact of low vision,” said Dr. Sahel. “The setting looks like a street, with eight randomized courses and four levels of light (2, 7.5, 50, and 500 lux). Patients are instructed to follow a delimited path while avoiding touching obstacles such as a hose, a bin, or a letterbox.” The patients’ movements are recorded to provide data on parameters such as course completion time, number of collisions, and preferred walking speed. In addition, patients report on their feelings about the lighting (e.g. comfortable, annoying, unbearable). Up until September 2019, seven of the nine patients treated with voretigene neparvovec had completed at least a baseline and one post-treatment visit following a prespecified protocol (Figure 11).
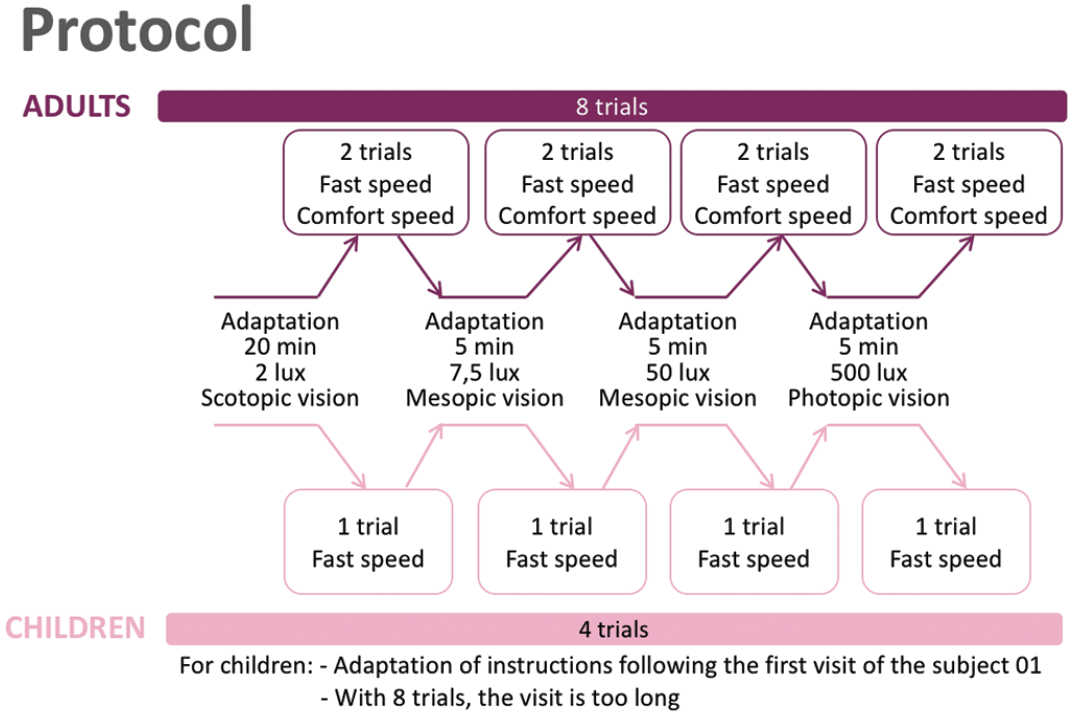
Figure 11. Streetlab study protocol.
Initial results show that course completion time and collision number at 2 lux and 7.5 lux decreased significantly following treatment in both adults and children. At 50 lux, course completion time decreased significantly after treatment when the analysis included adults (fast speed) and children, and collision number decreased significantly when the analysis included only adults (fast and comfort speed). At 500 lux, course completion time decreased significantly after treatment when the analysis included adults (fast speed) and children. Future analyses will study the correlation between visual data (VA, visual function, and FST) and mobility data.
Safety results observed to date have been in line with those from the phase 3 study,6 with no product-related serious adverse events and no deleterious immune responses. Going forward, a post-authorization registry-based safety study will evaluate the long-term safety profile of voretigene neparvovec for 5 years post-administration in a real-world setting.
To watch the presentation by Dr. José-Alain Sahel at EURETINA 2019, please click here.
