Diabetes is a growing worldwide epidemic, affecting the working age population. The number of individuals affected with diabetes is expected to increase by around 48% between 2017 and 2045.9 A common microvascular complication of diabetes, diabetic retinopathy (DR) is the leading cause of blindness in working-age adults in the developed world.10,11 The advanced, vision-threatening stages of DR are proliferative DR (PDR) and diabetic macular edema (DME).10,11
In clinical trials of DR, the Early Treatment Diabetic Retinopathy Study (ETDRS) DR severity scale (DRSS) is used to grade the stage of DR and monitor change over time.12 The scale can also be used in clinical practice. “The DRSS gives us all the opportunity to carefully study and accurately record changes in the stages and severity of retinopathy,” said Prof. Adrian Koh. The DRSS ranges from level 10 (normal) to level 85 (advanced PDR) in 12 steps (Figure 5).

Figure 5. The DRSS.
The DRSS is a clinically relevant measure that correlates with functional anatomical outcomes, including change in VA and retinal thickness.13 An increase in DRSS level is associated with an increased risk of developing vision-threatening PDR or DME, and reduction in DRSS level is associated with improved VA and DME resolution.13 A two-step change on the DRSS is considered to be clinically relevant. In the ETDRS study, patients with two or more steps of progression over the first 4 years were 5.8 times more likely to develop PDR than those without.12
Patients with eyes at DRSS levels 47 and 53 are on the threshold between PDR and non-proliferative DR (NPDR) and are at high risk of developing PDR. In eyes with DRSS scores of 47 and 53 at baseline, 66 and 80%, respectively, will progress to PDR in 5 years without treatment.12,14
Historically, clinical approaches to managing DR have consisted of risk factor control to manage blood glucose and blood pressure in patients with NPDR15 and panretinal photocoagulation (PRP) in patients with PDR.16 These aimed to prevent the development or progression of DR/DME and prevent further loss of vision. Restoring diminished VA was not a realistic treatment goal.17
However, evidence from an increasing number of clinical trials now supports a disease-modifying effect for ranibizumab in DR. “On reflection, improvement in retinopathy severity at the same time as improvement of DME upon treatment with ranibizumab makes sense, because, after all, PDR is mediated by an upregulation and surge of VEGF,” said Prof. Koh. In the RISE and RIDE studies, over 75% of ranibizumab-treated patients with DRSS scores of 47 to 53 at baseline had a ≥ two-step improvement in DR up to 36 months (Figure 6).18 These patients were also three times less likely than patients treated with sham to have experienced a new PDR event by month 36 (11.9% versus 35.2%).18 “This includes vitreous hemorrhage, pre-retinal hemorrhage, and tractional retinal detachment. This is not something trivial,” said Prof. Koh. “There is a real and consistent effect.” Overall, compared with patients in the sham arm, those treated with ranibizumab were significantly more likely to improve by ≥ 2 (5.4 vs 35.9%) or ≥ 3 (1.3 vs 14.5%) steps on the DRSS (both P < .001 vs sham).19
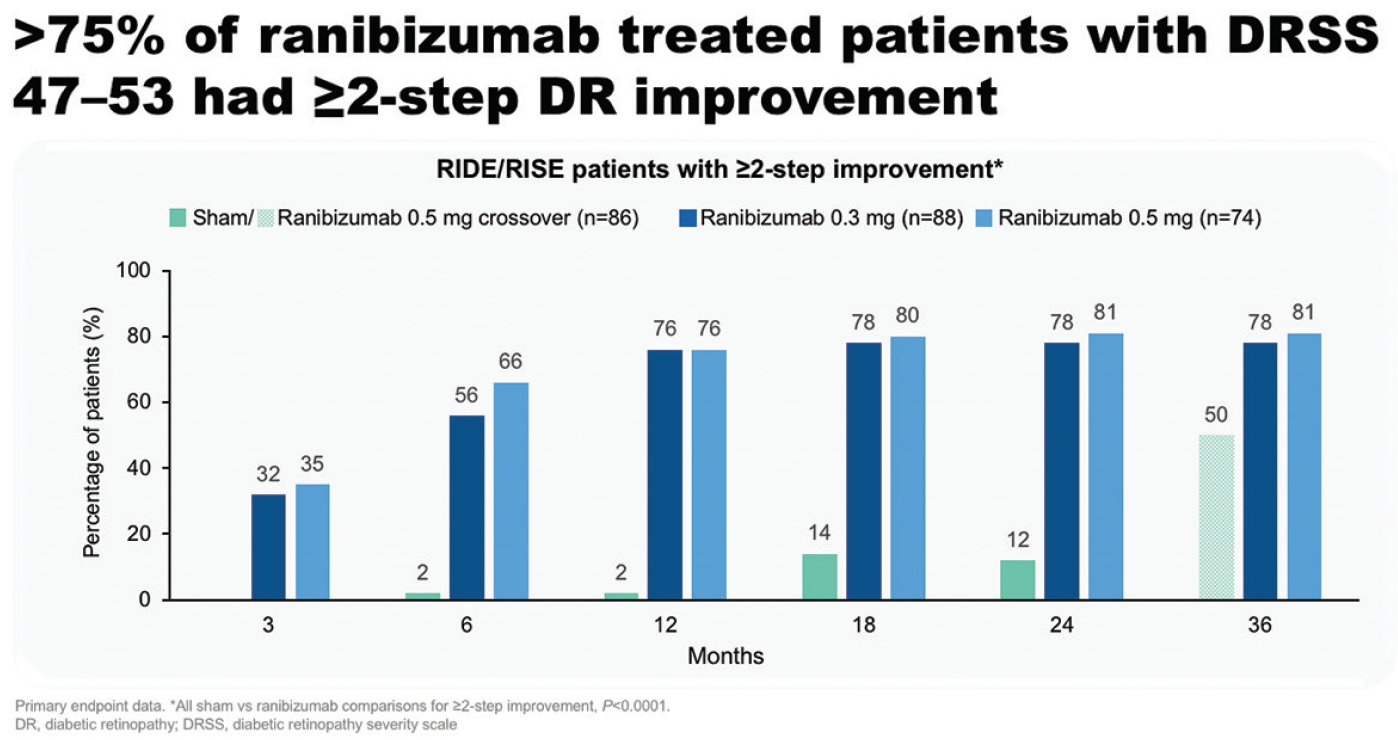
Figure 6. Patients with DRSS 47 to 53 with a ≥ two-step DR improvement in RISE and RIDE.
In the DRCR.net Protocol S study, patients with PDR treated with ranibizumab had lower rates of developing vision-impairing DME and less visual field loss at 5 years than those treated with PRP. In eyes without DME at baseline, 22% of patients treated with ranibizumab had developed DME at 5 years, compared with 38% of patients treated with PRP (Figure 7).20 PDR eyes both with and without DME at baseline achieved VA gains at 2 years with ranibizumab: 7.9 and 1.8 letters, respectively, compared with 1.9 and -0.5 letters for PRP.21
Highlights
- Ranibizumab treatment results in significant improvement of ≥ 2 or ≥ 3 DRSS steps in patients with DR and a reduced risk of DR worsening in eyes with or without PDR.19
- Better VA outcomes are achieved with ranibizumab monotherapy versus PRP in patients with and without DME.21
- Ranibizumab treatment consistently shows disease modifying activity in patients with DR.19-24
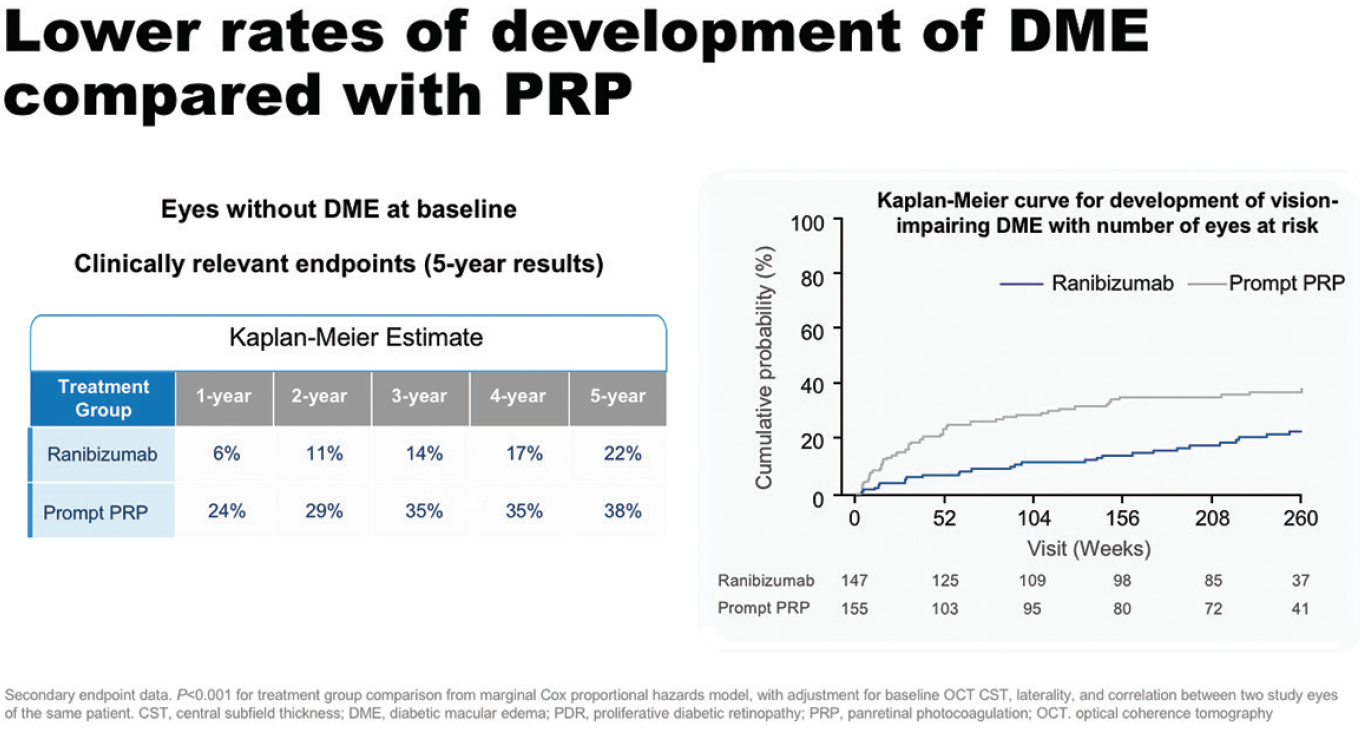
Figure 7. Development of DME in eyes without DME at baseline in Protocol S.
The DRCR.net Protocol I study demonstrated that ranibizumab treatment is associated with a reduced risk of DR worsening at 3 years compared with laser in eyes with or without PDR at baseline. This difference was significant in eyes without PDR at baseline (P = .01).22 Most recently, the PRIDE study compared ranibizumab alone or in combination with PRP with PRP alone in patients with PDR. At 12 months, patients treated with ranibizumab alone had a greater reduction in the area of neovascularization compared with those in the combination or PRP arms (Figure 8).23,24 In addition, more patients in the ranibizumab arm demonstrated complete regression of leakage from neovascularization at month 12 (28 vs 8% for PRP and 18% for combination).23,24
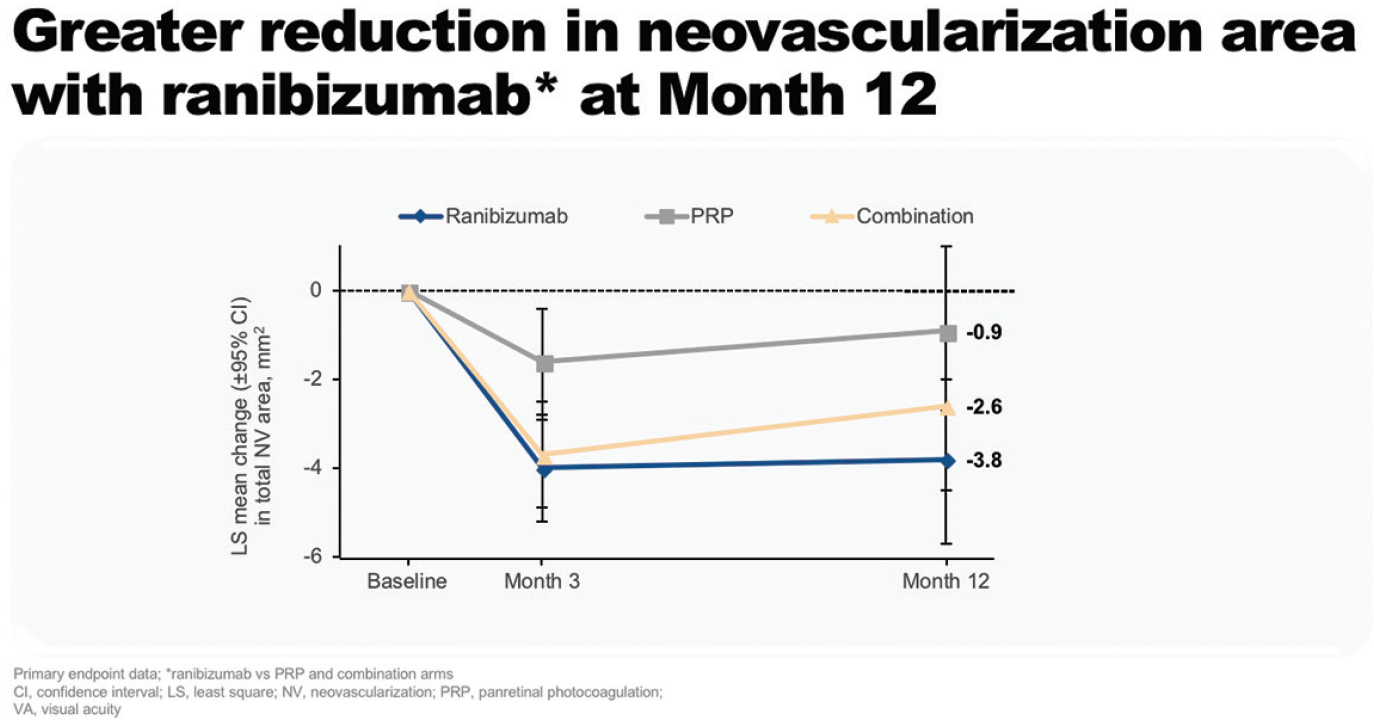
Figure 8. Reduction in neovascularization area at 12 months in PRIDE.
“There is compelling evidence across all trials for a disease-modifying effect of ranibizumab in DR. I believe that this marks the start of a paradigm shift in our approach to the management of PDR,” said Prof. Koh.

