Retinopathy of prematurity (ROP) is one of the most common complications of premature infants.25 It affects around 15 to 20% of all babies born preterm each year.26 “The incidence of ROP has increased in recent years as a result of advances in neonatal care resulting in increases in the numbers of premature births,”26,27 said Prof. Nicole Eter. ROP can be caused by a number of factors, including hypoxemia, postnatal oxygen supply, postnatal hyperglycemia, neonatal infections, and hypercarbia.28,29 Dysregulation of VEGF plays an important role in the development of ROP,30 leading to the hypothesis that anti-VEGF agents could be used in the treatment of ROP.
Clinical evidence for anti-VEGF therapy in ROP includes BEAT ROP, a study comparing bevacizumab with laser in 150 infants in the United States,31 and CARE ROP, a small study of 19 patients treated with 0.12 mg or 0.2 mg ranibizumab in Germany.32 Most recently, the international RAINBOW study compared ranibizumab 0.2 mg and 0.1 mg with laser in 225 patients with ROP.33,34
RAINBOW was a randomized, multicenter, open-label, parallel-group clinical trial to compare ranibizumab with laser therapy in premature infants with ROP (Figure 9).33,34 The primary objective was to demonstrate superior efficacy of ranibizumab 0.2 mg to laser as measured by treatment success at week 24. Treatment success was defined as the absence of the following criteria: death; the need for intervention for ROP with a treatment other than the assigned therapy; active ROP in either eye at week 24; and unfavorable structural outcomes (retrolental membrane obscuring the view of the posterior pole, substantial temporal retinal vessel dragging causing abnormal structural features/macular ectopia, or posterior retinal fold or retinal detachment involving the macula).33,34 Key secondary outcomes included demonstrating superior efficacy of ranibizumab 0.1 mg to laser and superior efficacy of ranibizumab 0.2 mg to ranibizumab 0.1 mg. Patients were male or female, with a birth weight of less than 1500 g and with bilateral ROP with one of the following categories of ROP in each eye: zone I, stage 1+, 2+, 3, or 3+ disease; zone II, stage 3+ disease; aggressive posterior ROP.33,34
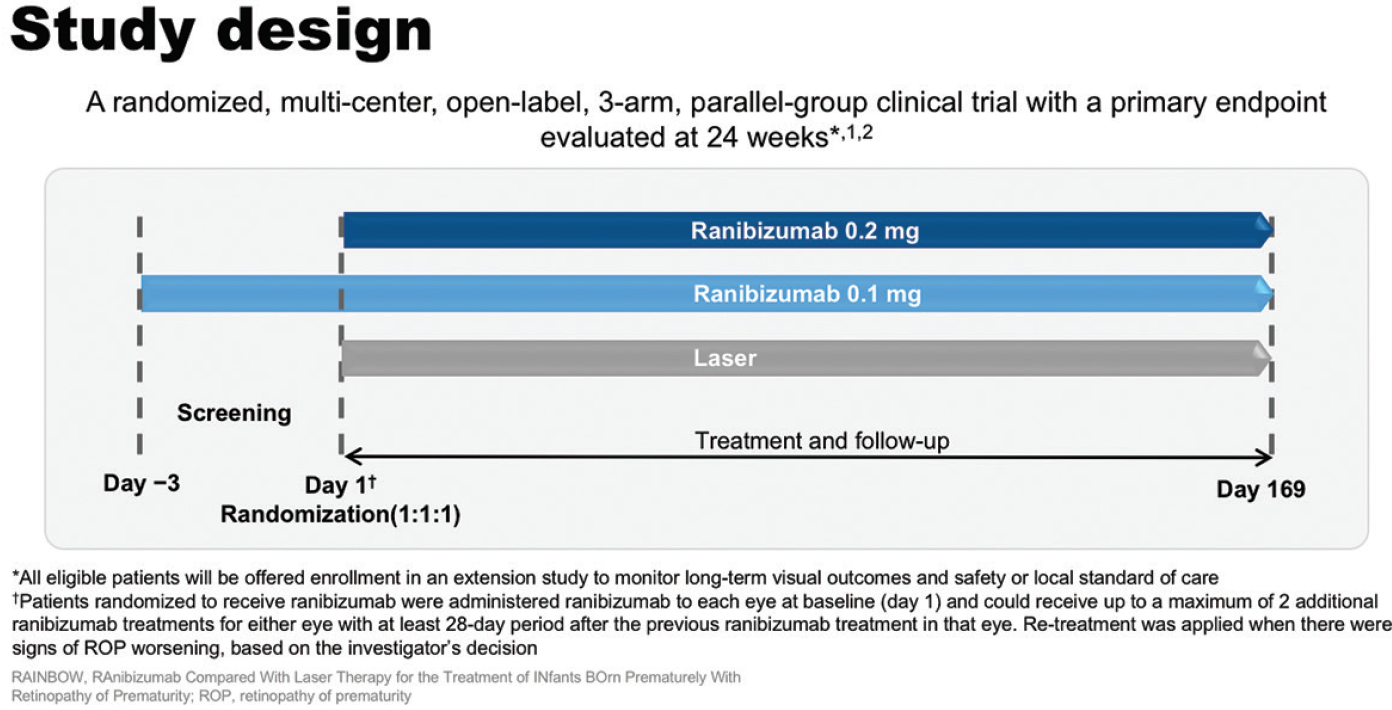
Figure 9. RAINBOW study design.
Of the 225 infants enrolled, 218 infants completed the study at week 24, including over 98% in each of the ranibizumab arms (Figure 10). Baseline characteristics were well balanced among the study groups, although the ranibizumab 0.2 mg group had the lowest mean birth weight (791 g vs 831 g in the laser arm). At baseline, most infants had zone II stage 3+ disease.33,34
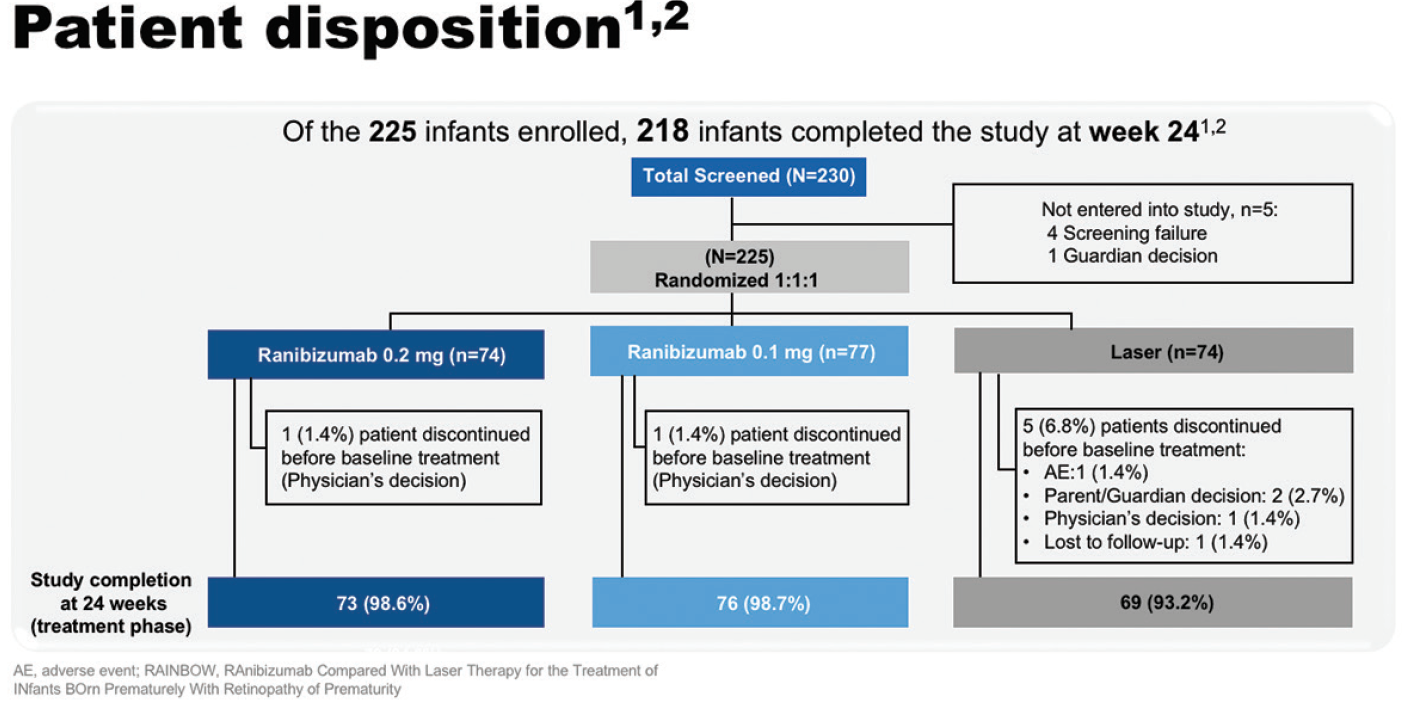
Figure 10. RAINBOW patient disposition.
On the primary outcome measure of treatment success at week 24, 80% of the infants achieved treatment success with ranibizumab 0.2 mg vs 66.2% with laser (odd ratio, 2.19; 95% CI, 0.99-4.82; one sided P = .0254; Figure 11).33,34 “Infants treated with ranibizumab 0.2 mg were twice as likely to achieve treatment success versus laser, which we consider to be clinically relevant,” said Prof. Eter. Higher treatment success was observed in patients with zone II ROP (88.1 vs 67.9% for zone II versus zone I in patients in the ranibizumab 0.2 mg group). The incidence of unfavorable structural outcomes was lowest in the ranibizumab 0.2 mg treatment group, with only one occurring during the course of the study, compared with seven in the laser arm.33,34
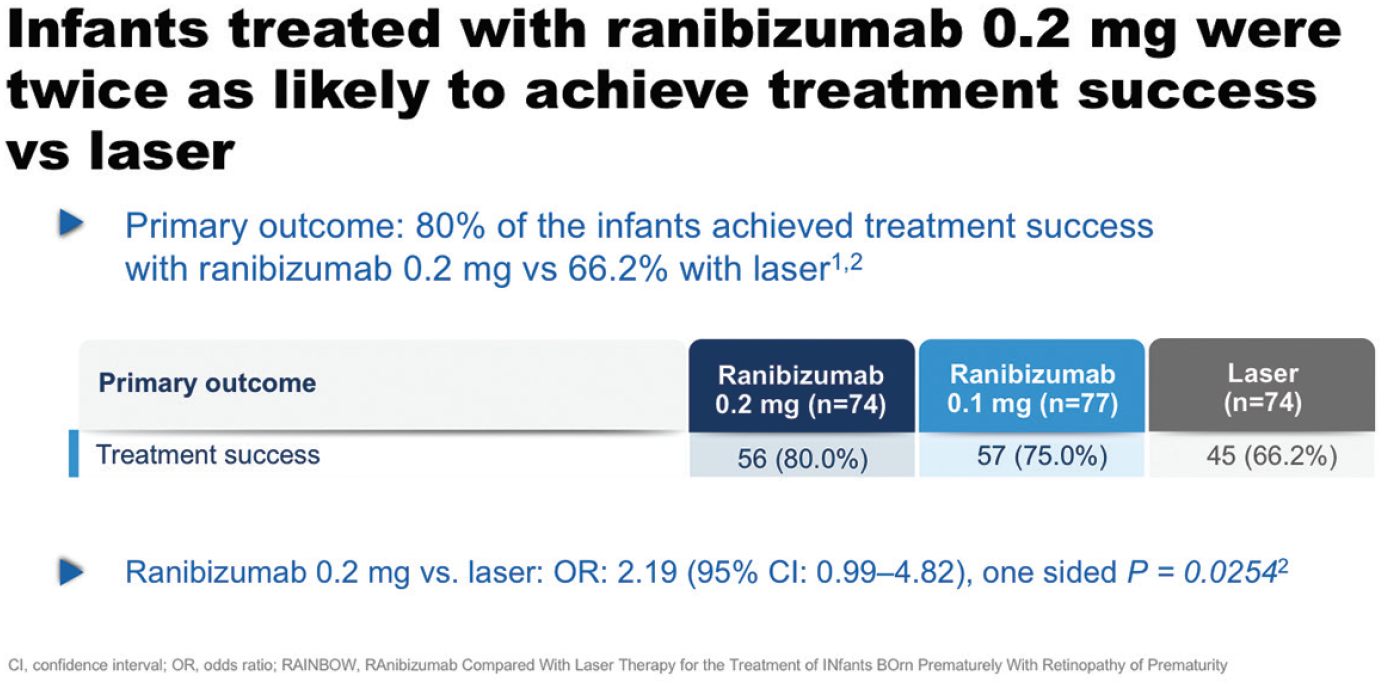
Figure 11. Primary outcome results in RAINBOW.
RAINBOW was the first study to report pharmacokinetic and systemic VEGF data in ROP. Serum ranibizumab levels were tested at day 1, 15, and 29, and the median concentration at day 29 was seven-fold lower compared with day 1 in the ranibizumab 0.2 mg group (a reduction from 7,820 pg/mL to 1,070 pg/mL). Systemic VEGF levels were tested on the same schedule, and across treatment groups there was a trend for a reduction in systemic VEGF concentrations between day 1 and 15, with return toward baseline by day 29.33,34
The frequency of ocular serious adverse events was low across all three groups. Non-ocular serious adverse events occurred in around one-third of patients, but this frequency was similar across groups and as expected in a preterm population.33,34
Based on the results of the RAINBOW study, on September 4, 2019, ranibizumab received approval from the European Commission for the treatment of ROP (zone I, stage 1+, 2+, 3, or 3+; zone II, stage 3+; or aggressive posterior ROP). “These are really exciting times for the youngest patients on ranibizumab treatment, the ROP babies,” said Prof. Eter.
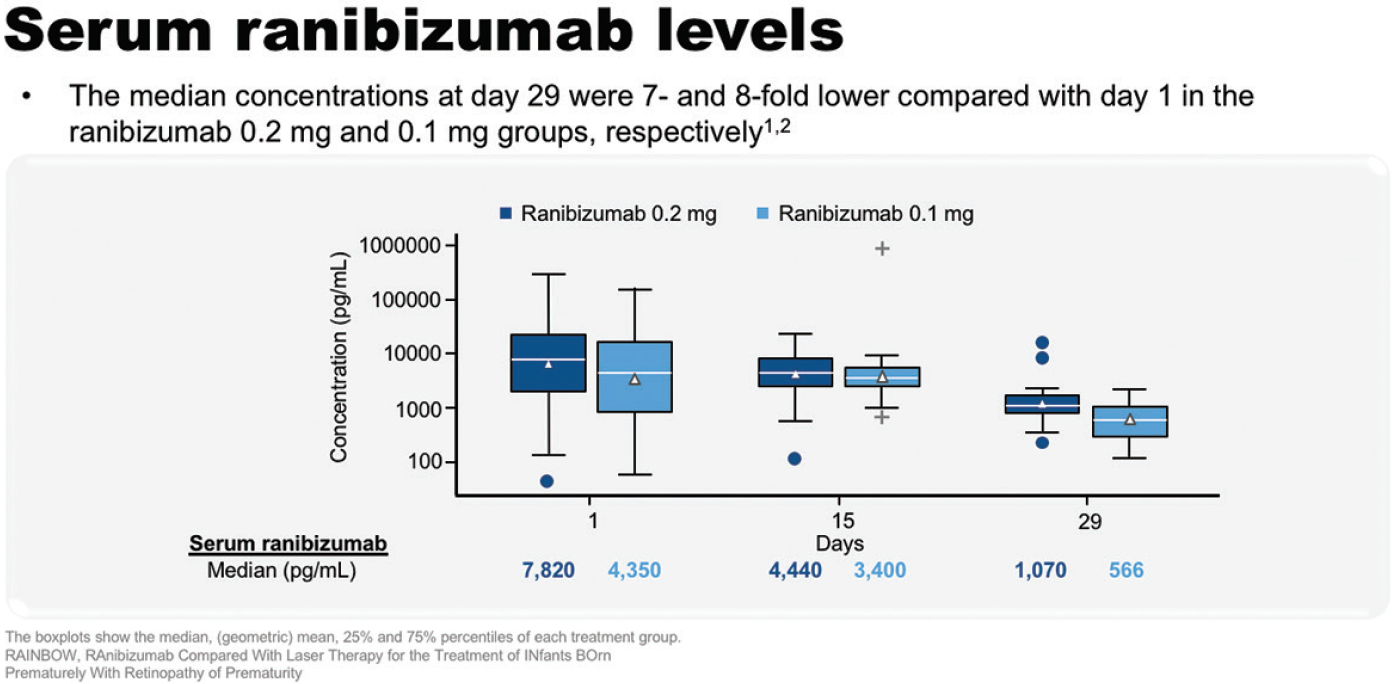
Figure 12. Serum ranibizumab levels in RAINBOW.
Highlights
- ROP is one of the most common avoidable causes of blindness, which is increasing in incidence with advances in neonatal care.25
- Approximately 15 to 20% of the estimated 15 million babies born preterm every year are affected with ROP, of whom up to 45,600 are diagnosed with irreversible visual impairment.26
- In the RAINBOW study, infants treated with ranibizumab 0.2 mg were twice as likely to achieve clinically relevant treatment success compared with those treated with laser.33,34
- Ranibizumab is now approved for the treatment of ROP in premature infants in Europe.

