Sponsored by Bausch + Lomb
Noninfectious posterior uveitis is a potentially blinding condition that requires vigilant treatment.1,2 This disease affects adults of all ages, with an estimated prevalence of 10 per 100,000 persons.2 Relentless placoid chorioretinitis (RPC), formerly known as ampiginous chorioretinitis, is a chronic posterior uveitis that was originally described by Jones et al. in 2000. 3-5 RPC resembles acute posterior multifocal placoid pigment epitheliopathy (APMPPE) or serpiginous choroiditis initially in the disease course.4,5 However, a relentless course beyond 6 months differentiates it from APMPPE.5 Additionally, retinal lesions in RPC have a distinctive retinal distribution in the mid and far periphery, unlike APMPPE or serpiginous choroiditis.4 The disease entity affects men and women equally and tends to appear between the 2nd and 6th decades of life.5 Visual symptoms often include blurred vision, metamorphopsia, paracentral scotomas, and/or floaters.5 The following case study describes a patient with RPC whose disease was slowly progressing in one eye despite systemic immunosuppression. The patient elected to receive RETISERT® (fluocinolone acetonide intravitreal implant) 0.59 mg therapy, which allowed the patient’s eye to maintain quiescence without the need for systemic therapy.
Case Report: Relentless Placoid Chorioretinitis
Background: A 30-year-old female patient was referred to my clinic with a diagnosis of posterior uveitis. Her symptoms included flashing lights, blurred vision, and scotomas in both eyes for the previous 5 to 6 months. She had prescription eyeglasses to correct for myopia and was generally healthy with no other systemic conditions or need for medications.
Diagnosis: The patient underwent a number of laboratory tests including quantiferon gold, rapid plasma reagin, and fluorescent treponemal antibody absorption, which were all negative and minimized the possibility of infectious uveitis due to tuberculosis or syphilis.6 Additionally, chest x-rays were normal, minimizing the possibility of sarcoidosis.7 A baseline eye examination indicated that her vision was 20/20 in the right eye with an IOP of 16 mm Hg. The vision in her left eye was 20/40 with an IOP of 15 mm Hg. Examination of the anterior segment was unremarkable, with a clear lens, and no detectable anterior chamber cells. However, there were trace cells visible in the vitreous.
Evaluation of the fundus revealed changes in the retinal pigment epithelium (RPE) and chorioretinal scarring in the macula in both eyes (Figure 1). OCT examination revealed RPE and outer retinal disruption in both eyes, with subfoveal involvement in the left eye (Figure 2), consistent with foveal lesions present in RPC.5 All clinical signs were suggestive of noninfectious posterior uveitis, and the patient was ultimately diagnosed with RPC.
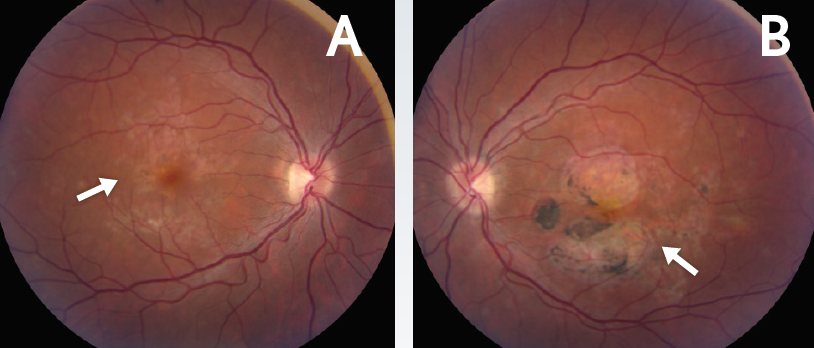
Figure 1. Chorioretinal scarring in the macula. Baseline fundus photos of the right (A) and left (B) eyes demonstrated RPE changes and chorioretinal scarring in the macula (arrows).
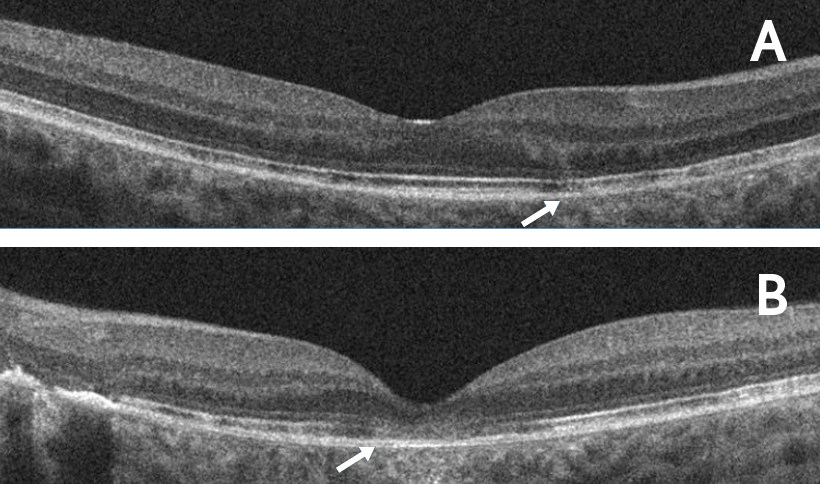
Figure 2. RPE and outer retinal disruption at baseline. OCT horizontal line scans of the right (A) and left (B) eyes demonstrated RPE and outer retinal disruption in both eyes (arrows). Subfoveal involvement was observed in the left eye.
Treatment: The patient began treatment with oral prednisone (60 mg daily) with a slow taper. During the taper, the patient experienced uveitis flare in both eyes. As a result, she was placed on a steroid-sparing immunomodulatory therapy (IMT) in addition to oral prednisone. The dose of steroid-sparing IMT was increased due to insufficient inflammatory control at the lower dose. After 1 year of therapy, the steroid-sparing IMT was discontinued due to elevation of liver enzymes and the need for intravitreal corticosteroid injections every 3 to 4 months. The patient then began another steroid-sparing IMT in place of the previous one. She continued to experience persistent disease activity, and there was a slow enlargement of the chorioretinal lesions. As a result, adalimumab therapy at 40 mg SQ every 2 weeks was added to her treatment regimen. She remained on this treatment regimen for 3 years and required approximately 1 to 2 intravitreal corticosteroid injections per year to maintain disease quiescence. Additionally, she developed cataracts in both eyes from the multiple intravitreal corticosteroid injections and required surgery for cataract removal.
After 4 years of her initial presentation and systemic therapy, the patient’s vision was maintained. Her VA was 20/20 in the right eye and 20/30 in the left eye. However, ultrawide-field imaging revealed new chorioretinal lesions in the right eye and enlargement of the chorioretinal lesion in the left eye (Figures 3A-B). Autofluorescence photos confirmed disease progression in both eyes as areas of hypoautofluorescence with areas of speckled hyperautofluorescence were visible (Figure 3C-D). In the left eye, it was apparent that atrophy was progressing and threatening the fovea (Figure 3D). OCT imaging demonstrated stable RPE and outer retinal disruption in right eye (Figure 4A). In the left eye there was a progression of outer retinal and RPE atrophy extending close to the fovea (Figure 4B).
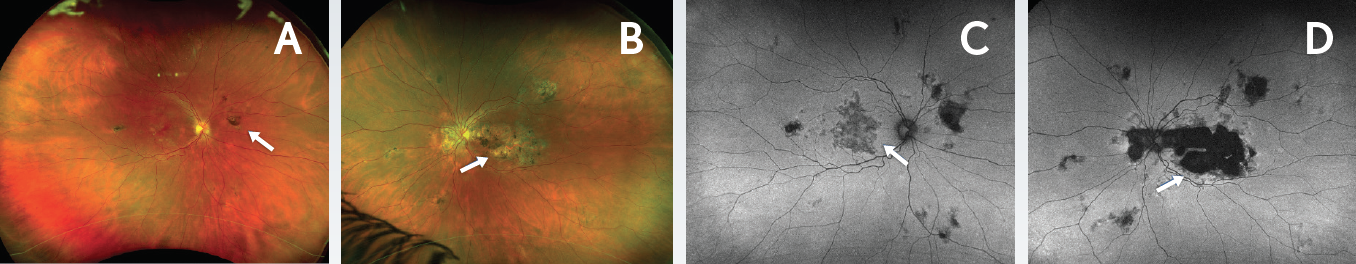
Figure 3. Disease progression 4 years after presentation. Ultrawide-field Optomap images demonstrated new chorioretinal lesions in the right eye (A, arrow) and enlargement of the chorioretinal lesion in the left eye (B, arrow). Autofluorescence images revealed areas of hypoautofluorescence with areas of speckled hyperautofluorescence in the right (C) and left (D) eyes (arrows). Autofluorescence of the left eye (D) indicated progression of atrophy that was threatening the fovea.
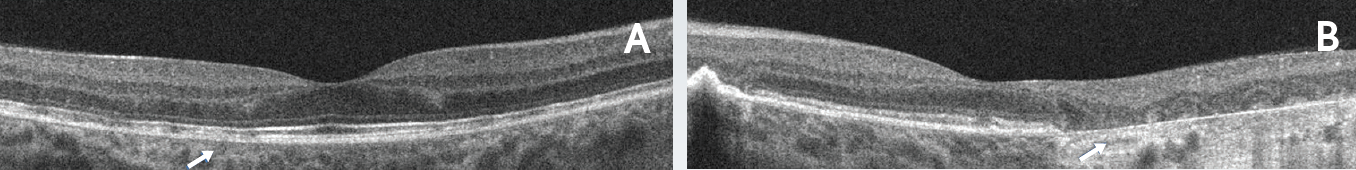
Figure 4. Outer retinal disruption and progression of RPE atrophy 4 years after presentation. Horizontal line scans of the right (A) and left (B) eyes. The right eye demonstrated stable RPE and outer retinal disruption. The left eye demonstrated progression of outer retinal and RPE atrophy extending close to the fovea.
Why RETISERT? The slow progression of chorioretinitis despite immunosuppression was threatening the fovea in the left eye and required long-term control. Over the years, the patient’s disease had been responsive to intravitreal corticosteroids, thus making her an appropriate candidate for a RETISERT implant, which delivers corticosteroid therapy over 2.5 years.8 The patient was concerned about side effects associated with systemic exposure while receiving systemic therapy.9 Thus, she was motivated to get off all systemic immunosuppression as she desired an alternate therapy that would deliver long-term control with minimal systemic exposure.
RETISERT is a corticosteroid implant indicated for the treatment of chronic noninfectious uveitis affecting the posterior segment of the eye.8 RETISERT is designed to release fluocinolone acetonide locally to the posterior segment of the eye with minimal systemic exposure.8 RETISERT is a viable alternative for patients with chronic conditions such as RPC who cannot tolerate or who do not desire to be on systemic corticosteroid therapy.10 The patient was counseled on the benefits and risks of RETISERT, including cataract development and IOP elevation, and elected to receive a RETISERT implant in her left eye.8 Since the patient was bilaterally pseudophakic at the time of RETISERT implantation, she was not at risk for RETISERT-induced cataract formation.
Patient Follow-up: After surgical implantation of RETISERT in the left eye, the patient’s vision was temporarily reduced from 20/30 to 20/40 on the first day after surgery and remained at 20/40 until 1 week after implantation. At 1 month following surgical implantation, her vision had returned to baseline (20/30). She was tapered off all systemic immunosuppression by 3 months following RETISERT implantation, and she didn’t receive topical corticosteroids in either eye. Chorioretinal atrophy was stable in her left eye at 3 months post-implantation (Figure 5). Her right eye experienced a flare 1 month after ending systemic immunosuppression therapy, and was treated with a local corticosteroid injection (Figure 6). Her right eye continues to be managed with intravitreal corticosteroid injections every 2 to 3 months.
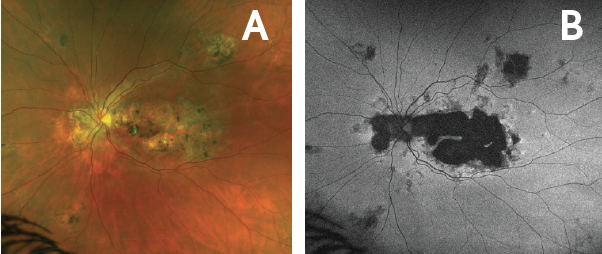
Figure 5. Quiescence in left eye at 3 months following RETISERT implantation. Ultrawide-field Optomap fundus photo (A) and autofluorescence photo (B) of the left eye 3 months following implantation demonstrated stabilization of chorioretinal atrophy. No progression was noted over 9 months post-implantation.
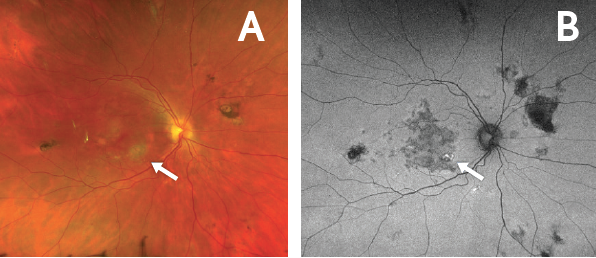
Figure 6. Flare in the right eye with discontinuation of systemic immunosuppression. Fundus photo of the right eye (A) demonstrated a new spot of active chorioretinitis (arrow). Corresponding autofluorescence photo (B) demonstrated hyperautofluorescence (arrow) 1 month after discontinuing systemic immunosuppression. The flare in this eye was managed with an intravitreal corticosteroid injection.
After 9 months of RETISERT therapy, the patient’s left eye was quiescent. IOP in the left eye had increased to 26 mm Hg approximately 4 months following implantation and was managed with dorzolamide hydrochloride/timolol maleate (2%/0.5%) ophthalmic solution BID. IOP in her left eye was 19 mm Hg at 9 months following RETISERT implantation.
Conclusions
This case study describes a patient who was diagnosed with RPC, a chronic bilateral noninfectious posterior uveitis that is characterized with lesions that are often found in the mid and far periphery with subsequent involvement of the posterior pole and/or macula.3,5 In the years following her diagnosis, the patient was treated with systemic immunosuppression, which did not provide adequate or consistent control of inflammation. Although she was able to maintain her vision, the chorioretinal lesions enlarged, and it was apparent that the disease was progressing. With a RETISERT implant in her left eye, the lesions had stabilized, and her eye was quiescent. The patient was able to achieve inflammatory control in both eyes with local corticosteroid therapy without the need for systemic immunosuppression.
INDICATION
RETISERT® (fluocinolone acetonide intravitreal implant) 0.59 mg is a corticosteroid indicated for the treatment of chronic noninfectious uveitis affecting the posterior segment of the eye.
IMPORTANT SAFETY INFORMATION
- Surgical placement of RETISERT® (fluocinolone acetonide intravitreal implant) 0.59 mg is contraindicated in active viral, bacterial, mycobacterial or fungal infections of the eye.
- Based on clinical trials with RETISERT®, during the 3‐year post‐implantation period, nearly all phakic eyes are expected to develop cataracts and require cataract surgery.
- As with any surgical procedure, there is risk involved. Potential complications accompanying intraocular surgery to place RETISERT® into the vitreous cavity may include, but are not limited to, the following: cataract formation, choroidal detachment, endophthalmitis, hypotony, increased intraocular pressure, exacerbation of intraocular inflammation, retinal detachment, vitreous hemorrhage, vitreous loss, and wound dehiscence.
- Following implantation of RETISERT®, nearly all patients will experience an immediate and temporary decrease in visual acuity in the implanted eye which lasts for approximately one to four weeks post‐operatively.
- Use of corticosteroids may result in elevated IOP and/or glaucoma. Based on clinical trials with RETISERT®, within 3 years post‐implantation, approximately 77% of patients will require IOP lowering medications to control intraocular pressure and 37% of patients will require filtering procedures to control intraocular pressure.
- Patients should be advised to have ophthalmologic follow‐up examinations of both eyes at appropriate intervals following implantation of RETISERT®. Physicians should periodically monitor the integrity of the implant by visual inspection.
- Ocular administration of corticosteroids has been associated with delayed wound healing and perforation of the globe where there is thinning of the sclera.
- The most frequently reported ocular adverse events in clinical trials with RETISERT® occurring in 50‐90% of patients included: cataract, increased intraocular pressure, procedural complications and eye pain. The most common non‐ocular event reported was headache (33%).
Click here for full Prescribing Information for RETISERT®.
RETISERT and the RETISERT READY logo are trademarks of Bausch & Lomb Incorporated or its affiliates.
© 2023 Bausch & Lomb Incorporated or its affiliates. All rights reserved.
RET.0032.USA.20_v3
1. Dick AD, Tundia N, Sorg R, et al. Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology. 2016;123(3):655-662.
2. Thorne JE, Suhler E, Skup M, et al. Prevalence of noninfectious uveitis in the United States: a claims-based analysis. JAMA Ophthalmol. 2016;134(11):1237-1245.
3. Jabs DA, Busingye J. Approach to the diagnosis of the uveitides. Am J Ophthalmol. 2013;156(2):228-236.
4. Jones BE, Jampol LM, Yannuzzi LA, et al. Relentless placoid chorioretinitis: a new entity or an unusual variant of serpiginous chorioretinitis? Arch Ophthalmol. 2000;118(7):931-938.
5. Raven ML, Ringeisen AL, Yonekawa Y, et al. Multi-modal imaging and anatomic classification of the white dot syndromes. Int J Retin Vitr. 2017;3:12.
6. Lin P. Infectious uveitis. Curr Ophthalmol Rep. 2015;3(3):170-183.
7. American Academy of Ophthalmology. Sarcoid uveitis. http://eyewiki.aao.org/Sarcoid_Uveitis. Updated October 3, 2019. Accessed February 11, 2020.
8. RETISERT [prescribing information]. Bausch & Lomb Incorporated.
9. Pasadhika S, Rosenbaum JT. Update on the use of systemic biologic agents in the treatment of noninfectious uveitis. Biologics. 2014;8:67-81.
10. Sharief LAT, Lightman S, Tomkins-Netzer O. Using local therapy to control noninfectious uveitis. Ophthalmology. 2018;125(3):329-331.


